Oligo-Antibody Conjugates
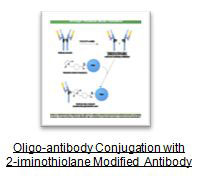
Antibody molecules possess a number of functional groups suitable for modification or conjugation purposes. Cross-linking oligonucleotide-antibodies can be achieved through lysine ϵ-amine and N-terminal α-amine groups. Carboxylate groups also may be used to couple with another molecule using the C-terminal end as well as aspartic aid and glutamic acid residues. Although amine and carboxylate groups are as plentiful in antibodies as they are in most proteins, the distribution of these functional groups is nearly uniform on the antibodies’ surface. For this reason, if some of the modified or conjugated residues are located on the antigen binding sites, the method may produce partially active or inactive oligonucleotide-antibody conjugates that may not bind to the antigen. In such cases, an alternative conjugation method using a thiol reactive group by selectively cleaving an antibody with a reducing agent to create two half-antibody molecules, or using smaller antibody fragment such as Fab'. Conjugation done using hinge area-SH groups will orient the attached oligonucleotide away from the antigen binding regions, thus preventing blockage of these sites and preserving activity.
The second alternative method of site-directed conjugation of antibody molecules
takes place at carbohydrate chains, typically attached to the CH2 domain within the
Fc region. Upon periodate oxidation, an aldehyde group can be introduced to the antibody,
which will allow it to react with an amine modified oligonucleotide.

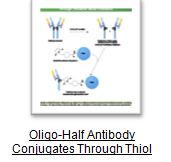
Oligo-Half Antibody Conjugates
Antibody is reduced at the hinge region to yield two half-antibody molecules, which then react with maleimide-activated oligos.
Price
The price varies based on the project specifications. Our service includes materials and labor for conjugation only! Price does not include the cost of biopolymer synthesis or purchase from a commercial vendor. If deemed necessary, biopolymer modification introduce additional functional groups, extra linkers or spacers will be an additional cost. Please contact us for a quote.
Service Description
Up to 3 mgs of starting material and 1-2 mgs of antibody for initial pilot conjugationprior production, is required for a thorough optimization, and method development
of purification and analysis. We have been successful with as little as 200 micrograms in some cases. We provide a few test runs for our customers to test.The test conjugates are agreed upon within one week. Bio-Synthesis will also provide a scale up production. After conjugation, a standard desalting, or purification, a small percent of heterogeneous products containing single or multi-site conjugate per molecule.
Specification
Depending on project specification, a pool of heterogeneous products in a small percentage may exist.
Procedure
Oligo synthesis and modification are manufactured under a strict, quality control process. Analytical HPLC and MS analyses are performed in every development cycle. After activating amine modified-oligos with NHS ester-maleimide, followed by gel filtration to remove excess cross-linking reagents, reduction of the antibody is followed by the conjugation of the antibody to maleimide-activated. Final target conjugates must first be isolated from excess or unreacted reagents. In many cases, simple dialysis removes unreacted reagents from the reaction solution (if the protein/antibody is significantly larger(>3-fold) than the modifying or coupling reagent). Additional purification such as stirred cell filtration, tangential flow filtration (TFF) or gel filtration chromatography may also be used to either remove excess reagents or isolate and characterize the cross-linked product. Reagents that are similar in size or larger than the antibody (mostly protein and other biological molecules) may require other purification techniques such as affinity chromatography, ion-exchange chromatography and hydrophobic interaction chromatography.
Cross-linked target molecules may then be further characterized by biochemical or biophysical techniques for an additional fee. Once the product has been purified, it may be subject to many different types of studies including spectroscopic (MALDI-TOF,ESI, LC-MS Fluorescence), electrophoresis, immunochemical biochemical and/or enzymatical analysis. QC (quality control) and QA (quality assurance) procedures are also followed independently to offer you double guarantee for the highest quality possible of every delivered conjugate. Moreover, our dedicated, technical account managers will guide your project through every step of the process, and constantly keep you informed of the latest project progress.
Disclaimer:
We can chemically link the intended components of a conjugated molecule. However, there exists the possibility that the binding sites/active sites of the protein can be altered/modified (partially or completely) independent of the stoichiometry used. Sometimes, this loss of activity is caused by physically blocking the antigen binding sites during conjugation, or by conformational changes in the complement-determining regions. Some proteins/antibodies are just too labile to undergo chemical modification reactions, regardless of the coupling methods used.
Bio-Synthesis can only guarantee the structure of our conjugates but not the suitability to specific biological applications.
Ordering and Submitting Requests for Bioconjugation Services
For us to better understand your customized project, please complete our Bioconjugation Service Questionnaire. The more our chemists understand your project’s needs, the more accurate your provided feedback will be. Providing us with your project’s details enables us to recommend the best reagents to use for your project. The most useful and readily available tools for bioconjugation projects are cross-linking reagents. A large number of cross-linkers, also known as bifunctional reagents, have been developed. There are several ways to classify the cross-linkers, such as the type of reactive group, hydrophobicity or hydrophilicity and the length of the spacer between reactive groups. Other factors to consider are whether the two reactive groups are the same or different (i.e. heterobifunctional or homobifunctional reagents), spacer is cleavable and if reagents are membrane permeable or impermeable. The most accessible and abundant reactive groups in proteins are the ϵ-amino groups of lysine. Therefore, a large number of the most common cross-linkers are amino selective reagents, such as imidoesters, sulfo-N-hydroxysuccinimide esters and N-hydroxysuccinimide esters. Due to the high reactivity of the thiol group with N-ethylmaleimide, iodoacetate and a-halocarbonyl compounds, new cross-linkers have been developed containing maleimide and a-carbonyl moieties. Usually, N-alkylmaleimides are more stable than their N-aryl counterparts.
In addition to the reactive groups on the cross-linkers, a wide variety of connectors and spacer arms have also been developed. The nature and length of the spacer arm play an important role in the functionality. Longer spacer arms are generally more effective when coupling large proteins or those with sterically protected reactive side-chains. Other important considerations are the hydrophobicity, hydrophilicity and the conformational flexibility. Long aliphatic chains generally fold on themselves when in an aqueous environment, making the actual distance spanned by such linker arms less than expected. Instead, spacers containing more rigid structures (for example, aromatic groups or cycloalkanes) should be used. These structures, however, tend to be very hydrophobic which could significantly decrease the solubility of the modified molecules or even modify some of their properties. In such cases, it is recommended to choose a spacer that contains an alkyl ether (PEO) chain. Bio-Synthesis offers several cross-linkers with PEO chains, such as thiol-binding homobifunctional reagents, heterobifunctional bases and their derivatives.
Within 3-5 days upon receiving your project scope, we will provide you an appropriate quotation. An order can be placed with PO (Purchase Order) or major credit cards (  ). Your credit card will be billed under Bio-Synthesis, Inc.
). Your credit card will be billed under Bio-Synthesis, Inc.