Bio-Synthesis offers a number of bioorthogonal reactions that can be use for detection, tracking and imaging purposes. Our services include but not limited to: click chemistry, Staudinger ligation, hydrazide-aldehyde reactions, aminoxy-aldehyde reactions and others.
Contact us to start your Bioorthogonal Probes
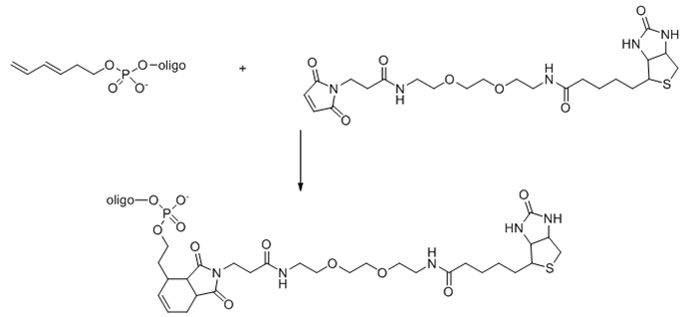
Diel-Alder Reaction
Diel-alder reaction consists of a 4 + 2 cycloaddition between a diene and an alkene,
often called dienophile. Diene modified oligonucleotides can be prepared by solid-phase
synthesis using a 3,5-hexadiene phosphoramidite derivative, which could be incorporated
into the oligo at the 5' end.
|
Modification
|
5'
|
Int
|
3'
|
Purification
|
|
Diene-modified oligonucleotides
|
Y
|
|
|
Dual HPLC
|
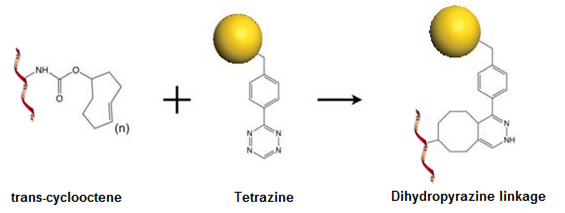
Tetrazine TCO Ligation Chemistry
The Trans-Cyclooctene-Tetrazine click chemistry is a very powerful tool in catalyst‐free
bioconjugation chemistry. The reaction follows an inverse‐electron demand Diels‐Alder
cycloaddition reaction of trans‐cyclooctenes (TCO) with tetrazines. This bioorthogonal
reaction possesses an exceptionally high reaction speed and nearly quantitative
yield under physiocological conditions with reaction rate constants 2000 M-1 s−1
(in 9:1 methanol/water). The extremely fast kinetics and selectivity enables the
conjugation of two low abundance biopolymers in an aqueous and otherwise complex
chemical environment through the formation of a stable dihydropyridazine. This bioorthogonal
reaction possesses extreme selectivity and biocompatibility, such that the complimentary
reagents can form covalent bonds within richly functionalized biological systems,
in some cases, living organisms.
|
Modification
|
5'
|
Int
|
3'
|
Purification
|
|
TCO-PEG4 NHS
|
Y
|
Y
|
Y
|
Dual HPLC
|
|
TCO-PEG3 NHS
|
Y
|
Y
|
Y
|
Dual HPLC
|
|
TCO-PEG12 NHS
|
Y
|
--
|
--
|
Single HPLC
|
|
UV-Tacer TCO NHS
|
Y
|
Y
|
Y
|
Dual HPLC
|
|
Tetrazine-PEG4 NHS
|
Y
|
Y
|
Y
|
Dual HPLC
|
|
Tetrazine-PEG5 NHS
|
Y
|
Y
|
Y
|
Dual HPLC
|
Hydrazine Aldehyde Reaction
The reaction between an aldehyde or ketone and a hydrazide or hydrazine functional
group to form a hydrzone bond has been frequently used for bioconjugation reaction.
Aldehyde, hydrazide modified oligonucleotides can be prepared by solid-phase synthesis
or functionalized post-synthetically.
|
Modification
|
5'
|
Int
|
3'
|
Purification
|
|
Aldehyde
|
Y
|
|
|
HPLC
|
|
Hydrazide
|
Y
|
Y
|
Y
|
Dual HPLC
|
Aminooxy Aldehyde Reaction
The chemoselective conjugation of an aminooxy modified oligo with an aldehyde is
similar to that of the reaction of a hydrazide with an aldehyde, except instead
of giving a hydrazone bond it yields an oxime linkage. Aminooxy modified oligonucleotides
can be prepared by solid-phase synthesis using a 5'-aminooxy modifier 11. This modifier
is based on a tetraethylene glycol linkage for improved solubility and for reducing
the potential negative impact on hybridization of the oligo. The oxime formed from
the reaction of alkyloxyamines with aldehydes creates a stable covalent bond.
|
Modification
|
5'
|
Int
|
3'
|
Purification
|
|
5' aminooxy 11 modification
|
Y
|
|
|
HPLC
|