Yes, we do.
We at Bio-Synthesis routinely use fluorenylmethyloxycarbonyl chloride (Fmoc-Cl) based solid phase peptide synthesis (SPPS) chemistry for the automated synthesis of peptides. We also use solution phase peptide synthesis in cases where a more complex peptide synthesis approach is needed.
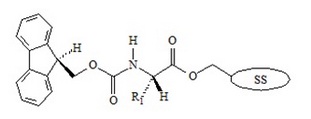
Improvements made in automated Fmoc-solid phase peptide synthesis (SPPS) during recent years now allows scientists, researchers and peptide companies to routinely assemble synthetic peptides containing 100 amino acids or more within the polypeptide chain. The Fmoc-solid phase peptide synthesis approach enables the synthesis of peptides, by coupling the carboxyl group of the incoming amino acid to the N-terminus of the growing peptide chain.
Because amino acids have multiple reactive groups, to facilitate peptide formation with minimal side reactions, amino acids with special protecting groups are used to avoid side reactions. These side reactions can reduce the length of the synthesized peptide and prevent nonspecific side reactions during synthesis. Commonly used side chain protection groups are the benzyl (Bzl) and tert-butyl (tBu) group. However, the use of a specific protecting group selected during the solid phase peptide synthesis for a given peptide can vary depending on the peptide sequence and the type of N-terminal protection employed.
Side protecting groups are also known as "permanent" protecting groups, because they can withstand multiple cycles of chemical treatment during each synthesis cycle. However, they can be selectively removed using special chemical treatment after the completed synthesis. During SPPS, the N-termini of amino acids are protected by groups that "temporarily" block the amino group with a specific protecting group. After successful coupling of the incoming Fmoc-amino acid during a cycle of the Fmoc-solid phase peptide synthesis or Fmoc-SPPS the Fmoc protecting group can be easily removed to allow peptide bond formation during the next cycle.
Two common N-terminal protecting groups are: tert-butoxycarbonyl (Boc) and fluorenylmethyloxycarbonyl chloride (Fmoc). Each of these two groups has distinct characteristics that determine their use. The Boc protecting group requires a moderately strong acid such as trifluoracetic acid (TFA) to be removed from the newly added amino acid, while the Fmoc protecting group is a base-labile protecting group that is removed with a mild base such as piperidine.
Because multiple protecting groups are normally used in peptide synthesis, it is evident that these groups must be compatible to allow deprotection of distinct protecting groups, while not affecting other protecting groups. This is called an orthogonal protecting and deprotecting strategy, which allows matching of protecting groups so that deprotection of one protecting group does not affect the stability of the other groups. Because N-terminal deprotection occurs continuously during solid phase peptide synthesis, protecting schemes have been established in which the different types of side chain protecting groups (Bzl or tBu) are matched to either Boc or Fmoc SPPS, respectively, for optimized deprotection.
10/13