Methylphosphonate oligonucleotides carry a methyl group at their internucleotidic linkages. These internucleotide modifications were first proposed by Miller and Tso (1) and were specifically intended to address characteristics desirable for therapeutic use of oligonucleotides. It had been widely observed that highly charged therapeutic molecules, even small molecules like nucleoside mono-phosphates, are not taken up by cells in culture or by animal tissues.
The nonionic methylphosphonates are resistant to enzymatic degradation, they have the ability to enter target cells, show a lack of interference with normal DNA and RNA processing enzymes, and have the ability to bind to a target and alter its expression in a sequence specific manner.
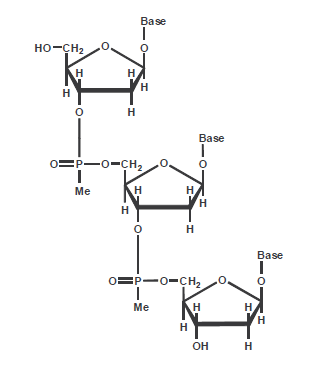
The rationale for the methylphosphonate modification was that removing the negatively charged oxygen from the internucleotide bond and replacing it with a non-charged methyl group should increase the ability of these molecules to be taken up by cells in culture and simultaneously protect the resulting modified DNA from being degraded by phosphodiesterases.
What was observed with this modification is that by removing a required recognition element from the DNA mimic, i.e., the negatively charged backbone, the resulting oligonucleotides become inactive in many biological roles.
The internucleotide bond in methylphosphonate DNA is highly resistant to nucleases, and reported to give enhanced cellular uptake in various assays. The modification became more broadly accepted when it was demonstrated that it could be incorporated using “phosphoramidite-like” monomers on a typical DNA synthesizer and is now most often incorporated along with natural internucleotide bonds (2) at 3' and or 5' ends of DNA in antisense experiments. Due to their low solubility and product yield, a maximum of 3 methyl-phosphonate linkage on either end of your DNA should not be exceeded.
Advantages
Disadvantage
- low solubility in aqueous solutions
- low porduct yield
Application
References
- Miller, P. S., McParland, K. B., Jayaraman, K., Tso, P., Biochemistry, 1981, 20, 1874-1880.
- Hogrefe, R. I., Vaghefi, M. M., Reynolds, M. A., Young, K. M., Arnold L. J., Nucleic Acids Research, 1993, 21, 2031-2038.