The amino acid acivicin [(alpha S,5S)-alpha-amino-3-chloro-4,5-dihydro-5-isoxazoleacetic acid; AT-125; NSC-163501] is a fermentation product of Streptomyces sviceus. Acivicin is a modified amino acid, a glutamine analog that irreversibly inhibits glutamine-dependent amidotransferases known to be a potent γ-glutamyl transpeptidase inhibitor that has been used to elucidate aspects of glutathione metabolism and has known anti-tumorigenic activity. This amino acid inhibits tumor growth in cell lines dependent on glutamine metabolism. Glutamine-dependent amidotransferases are enzymes that are involved in nucleotide and amino acid biosynthesis active in a variety of mouse tumor models including the L1210 and P388 leukemias, the M5076 ovarian carcinoma, and the MX-1 human breast tumor xenograft. It is thought that antitumor activity is mediated through the inhibition of enzymes that catalyze the transfer of an amido group from L-glutamine, especially the enzymes cytidine triphosphate (CTP) synthetase and xanthosine-5'-phosphate:ammonia ligase (XMP) aminase. CTP synthetase is an enzyme involved in pyrimidine biosynthesis that interconverts Uridine-5'-triphosphate (UTP) and Cytidine triphosphate (CTP). XMP aminase or GMP synthase (EC6.3.4.1, xanthosine-5'-phosphate-ammonia ligase, guanylate synthetase, xanthosine 5'-monophosphate aminase) is an enzyme essential for the biosynthesis of nucleic acid guanine. Acivicin is known to have anticancer and antitumor activity in vivo and to prevent melanoma metastasis in vitro.
Since this molecule is a modified non-typical amino acid it contains an amino group that allows the analysis with the help of standard amino acid analysis or pre- or post-column derivatization prior to the analysis using HPLC-UV or DAD detection. In addition, the non-derivatized amino acid me be detected using liquid-chromatography mass-spectrometry based methods as well.
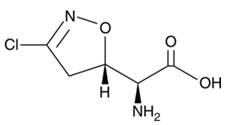
Acivicin [(2S)-Amino[(5S)-3-chloro-4,5-dihydro-1,2-oxazol-5-yl]ethanoic acid]; molecular formula: C5H7ClN2O3; molar mass: 178.57 g mol−1.
Williams et al. in 2009 solved the crystal structure of the acivicin-modified H.pylori γ-glutamyltranspeptidase (HpGT). The structure of acivicin-modified HpGT suggests the nucleophilic attack of Thr 380 Oγ at the C3 of acivicin and the displacement of chloride. Furthermore, the researchers report that the integrity of the dihydroisoxazole ring is likely maintained during the reaction since C3 retains its sp2 hybridization. The observed unique C-terminal capping of the active site within the acivicin-modified HpGT structure, together with mutagenesis studies, demonstrated that Phe 567 contributes to the overall catalytic efficiency of the enzyme. The researcher examined the contributions of residues within the C-terminal domain of the 20 kDa subunit and found that local structural motifs are critical for optimal enzymatic activity and autoprocessing. These new insights into the HpGT structure and function relationships are proposed to help facilitate the design of future selective γGT inhibitors. Figure 1 depicts the structure model of the acivicin-inhibited γ-glutamyltranspeptidase (γ-GT).
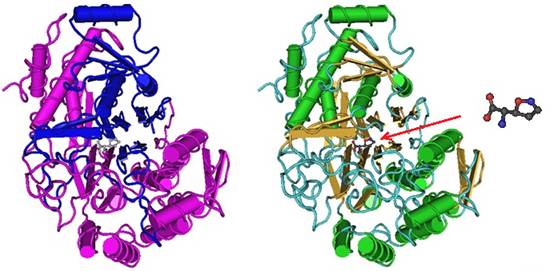
Figure 1: Structure model of the acivicin-inhibited γ-glutamyltranspeptidase (γ-GT). (Left) The domain structures are highlighted in different colors. (Middle) The secondary structures are highlighted in different colors. (Right) The structure of acivicin is shown and its location inside the protein binding pocket is indicated by the red arrow.
References
Poster DS, Bruno S, Penta J, Neil GL, McGovren JP.; Acivicin. An antitumor antibiotic. Cancer Clin Trials. 1981 Fall;4(3):327-30.
Williams K, Cullati S, Sand A, Biterova EI, Barycki JJ.; Crystal structure of acivicin-inhibited gamma-glutamyltranspeptidase reveals critical roles for its C-terminus in autoprocessing and catalysis. Biochemistry. 2009 Mar 24;48(11):2459-67. doi: 10.1021/bi8014955.