Amino Acid Analysis of Tyramine
By Klaus Linse
Amino acid analysis allows for the analysis and quantitative detection of tyramine using a standard amino acid analyzer. Amino acid analysis of Tyramine can be performed either with pre-column or post-column modification or labeling since this naturally occurring monoamine contains a primary amino group. In addition, this technique lends itself to quantitatively analyze tyramine levels in food or feed products.
Tyramine can trigger headaches and migraines in some individuals. People who take monoamine oxidase inhibitors or are especially sensitive to tyramine should eat a diet containing low amounts of this amine. High levels of tyramine have been found in aged foods such soy sauce, salami and sauerkraut. The solution to preventing migraines is to eat fresh foods and cut down on the use of prepared and processed foods.
Tyramine, 2-(4-Hydroxyphenyl)ethylamine, 4-(2-Aminoethyl)phenol, or 4-Hydroxyphenethylamine, is a naturally occurring monoamine and trace biogenic and sympathomimetic amine derived from the amino acid tyrosine. Tyramine has the formula HOC6H4CH2CH2NH2 , and a molecular weight of 137.18. Molecular identification numbers of tyramine are the CAS Number 51-67-2, the Beilstein Registry Number 1099914, the EC Number 200-115-8, the MDL number MFCD00008193, and the PubChem Substance ID 24900582.
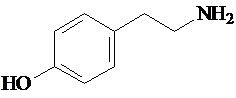
Figure 1: Tyramine Structure.
The biogenic amine tyramine can act as a catecholamine releasing agent. Catecholamines (CA) are monoamines, organic compounds that have a catechol group, which contain a benzene ring with two hydroxyl side groups and a side-chain amine. The term catechol refers to the 1,2-dihydroxybenzene group. Catecholamines are derived from the amino acid tyrosine and are water-soluble and approximately 50% of catecholamines are bound to plasma proteins when they circulate in the bloodstream. Tyramine by itself is unable to cross the blood-brain barrier and causes only non-psychoactive peripheral sympathomimetic effects. Some of its effects resemble effects of epinephrines. However, a hypertensive crisis can result from ingestion of tyramine-rich foods together with monoamine oxidase inhibitors (MAOIs).
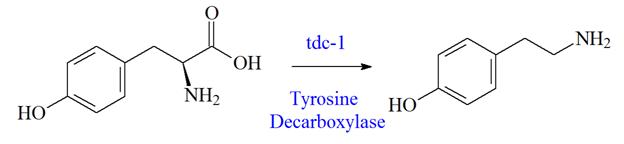
Tyrosine Tyramine
Figure 2: Tyrosine decarboxylation reaction. According to the wormatlas, tyrosine is converted to tyramine via a decarboxylation reaction by the enzyme tyrosine decarboxylase. Tyramine is further converted to octapamine by the enzyme tyramine β–hydroxylase.
Tyrosine is the precursor to catecholamines and tyramine is a breakdown product of tyrosine. In the gut and during fermentation, tyrosine is decarboxylated to tyramine. During normal metabolism in humans and mammals tyramine is deaminated in the liver to an inactive metabolite. David et al. in 1974 showed using MF1 mice that, as tissue concentrations of tyrosine are increased, decarboxylation becomes increasingly important and, at very high tissue levels, is the predominant route of metabolism. However, when the hepatic monoamine oxidase is inhibited, the clearance of tyramine is blocked and circulating tyramine levels can increase. Elevated levels of tyramine can compete with tyrosine for transport across the blood–brain barrier where it can then enter adrenergic nerve terminals.
Biogenic amines, including tyramine, are molecules that have allergenic properties and are often found in many fermented products. The decarboxylation of tyrosine during fermentation or decay in foods can lead to its presence in many food matrices. The amines are synthesized by lactic acid bacteria through the decarboxylation of amino acids present in the food matrix. However, the concentration of biogenic amines in fermented foodstuffs is influenced by many environmental factors. In addition, it is now known that peptides containing amino acids precursors of biogenic amines can be used by bacteria to produce these biogenic amines. For example, tyramine can be produced from peptides containing tyrosine and free tyrosine is not the only precursor for tyramine production. Tyramine, together with histamine, if present in wine can cause headaches and migranes. The lactobacillus plantarum IR BL0076 strain isolated from wines of the Rhône Valley during aging is known to produce tyramine. Some enological practices, the art of wine making, which lead to an enrichment in nitrogen compounds therefore favor biogenic amine production in wine. Furthermore, tyramine is found in many plants and animals. The molecule is metabolized by the enzyme monoamine oxidase. Tyramine is also present in ergot, mistletoe and putrefied animal matter. Foods that may contain tyramine include ripe cheeses, beers, red wines, meats that are potentially spoiled or pickled, aged, smoked, fermented, or marinated, pork, chocolate, alcoholic beverages, and many fermented foods, such as cheeses, sour cream, yogurt, shrimp paste, soy sauce, and many others. In general tyramine is produced in foods from the natural breakdown of the amino acid tyrosine or tyrosine containing peptides but is not added to foods. Tyramine levels can increase in aging foods, or foods that are fermented or stored for long periods of time. Some fermented foods, and especially cheese, are within the food products more often related with biogenic amines poisoning. During the last century, a close relation between migraine crisis and ingestion of tyramine-rich food, especially cheese, was observed and these effects of tyramine consumption were coined as cheese-reaction.
Tyramine plasma levels, together with plasma tyrosine and methionine levels are significantly elevated in individuals with type II tyrosinemia. This is caused by a deficiency of the enzyme tyrosine aminotransferase. The disorder can affect the eyes, skin, and mental development.
References
Maryse Bonnin-Jusserand, Cosette Grandvalet, Aurélie Rieu, Stéphanie Weidmann and Hervé Alexandre; Tyrosine-containing peptides are precursors of tyramine produced by Lactobacillus plantarum strain IR BL0076 isolated from wine. BMC Microbiology 2012, 12:199.
JEAN-CLAUDE DAVID, WALLACE DAIRMAN, AND SIDNEY UDENFRIEND; Decarboxylation to Tyramine: A Major Route of Tyrosine Metabolism in Mammals. Proc. Nat. Acad. Sci. USA Vol. 71, No. 5, pp. 1771-1775, May 1974.
Daniel M. Linares, Beatriz del Río,Victor Ladero, Noelia Martínez, María Fernández, María Cruz Martín and Miguel A. Álvarez; Factors influencing biogenic amines accumulation in dairy products. Frontiers in Microbiology. 2012, Volume 3, Article 180, 1-10.
http://www.biomedcentral.com/1471-2180/12/199.
http://www.wormatlas.org/neurotransmitterstable.htm