Dual labeled probes
Methods that use fluorescence are widely used in biological sciences including biochemistry, biophotonics, biophysics, cell biology, clinical chemistry, histochemistry, and molecular medicine. Molecules in electronically excited states emit light and fluoresce. Many biological molecules display intrinsic fluorescence, or can be labeled with molecules that exhibit fluorescence. Fluorescent molecules used as labels are also called fluorophores. Fluorescently labeled molecules are also called molecular probes. Typically, fluorescent molecules are aromatic compounds that display light absorption in the ultraviolet to visible regions of the electromagnetic spectrum. UV: 250 to 400 nm; Visible: 400 to 700 nm.
Fluorescently labeled probes are useful for many biochemical assays for monitoring of specific molecular events such as binding, cleavage or conformational changes of oligonucleotides, proteins, and peptides. Dual labeled probes containing a fluorophore and a quencher molecule have many applications in genetic analysis. Dual labeled probes are often used for quantitative polymer chain reaction (qPCR). Dual labeled probes are single-stranded oligonuclotides labeled with two different dyes, a fluorophore and a quencher molecule. Dual labeled probes are hybridization probes that can be synthesized using standard automated oligonucleotide chemistry and are also referred to as hydrolysis probes. Dual labeled probes function by reporting the presence of specific nucleic acids in homogenous samples in solution. Usually, a reporter dye is located at the 5’-end and a quencher molecule located at the 3’-end. The quencher molecule inhibits or decreases the fluorescence intensity of a sample or fluorophore via fluorescence resonance energy transfer (FRET). When the primer is elongated by the polymerase during PCR the dual labeled probe can bind to the amplified specific DNA template. Hydrolysis releases the reporter molecule from the probe or target hybrid causing an increase in fluorescence and the measured fluorescence signal is directly proportional to the amount of target DNA.
What is FRET?
Resonance energy transfer or RET occurs when an energy quantum is transmitted from its site of absorption to the site of its utilization in a molecule, or system of molecules. This phenomenon occurs between chromophores over distances greater than interatomic, without conversion to thermal energy, and without kinetic collision of the donor and acceptor.
The donor is the dye that initially absorbs the energy.
The acceptor is the chromophore or dye to which the energy is transferred.
Fluorescence resonance energy transfer or FRET is now widely used in many fluorescence based applications. In the past FRET has been widely used to measure dimensions and distances within or between molecules over distances of 10 to 100 Å. This distance range is well suited to probe structures of oligonucleotides, proteins and peptides. The range of applications includes medical diagnostics, DNA analysis as well as optical imaging and many others. Favorable distances for energy transfer are typically in the size of proteins, DNA or RNA or the thickness of a membrane. Furthermore, the extent of FRET is predictable from spectral properties of the selected fluorophors. These properties allow for the design of experiments based on the known sizes and structural features of the studied sample.
FRET is an electromagnetic phenomenon that can be explained using the laws of classical physics. FRET occurs between a donor (D) molecule in the excited state and an acceptor (A) molecule in the ground state. Typically, the donor molecule emits at shorter wavelengths that overlap with the absorption spectrum of the acceptor molecule. The energy transfer occurs without the appearance of a photon as a result of long-range dipole-dipole interactions between donor and acceptor. The extent of spectral overlap of the emission spectrum of the donor with the absorption spectrum of the acceptor, the quantum yield of the donor, the relative orientation of the donor and acceptor transition dipoles, and the distance between the donor and the acceptor molecule determine the rate of energy transfer.
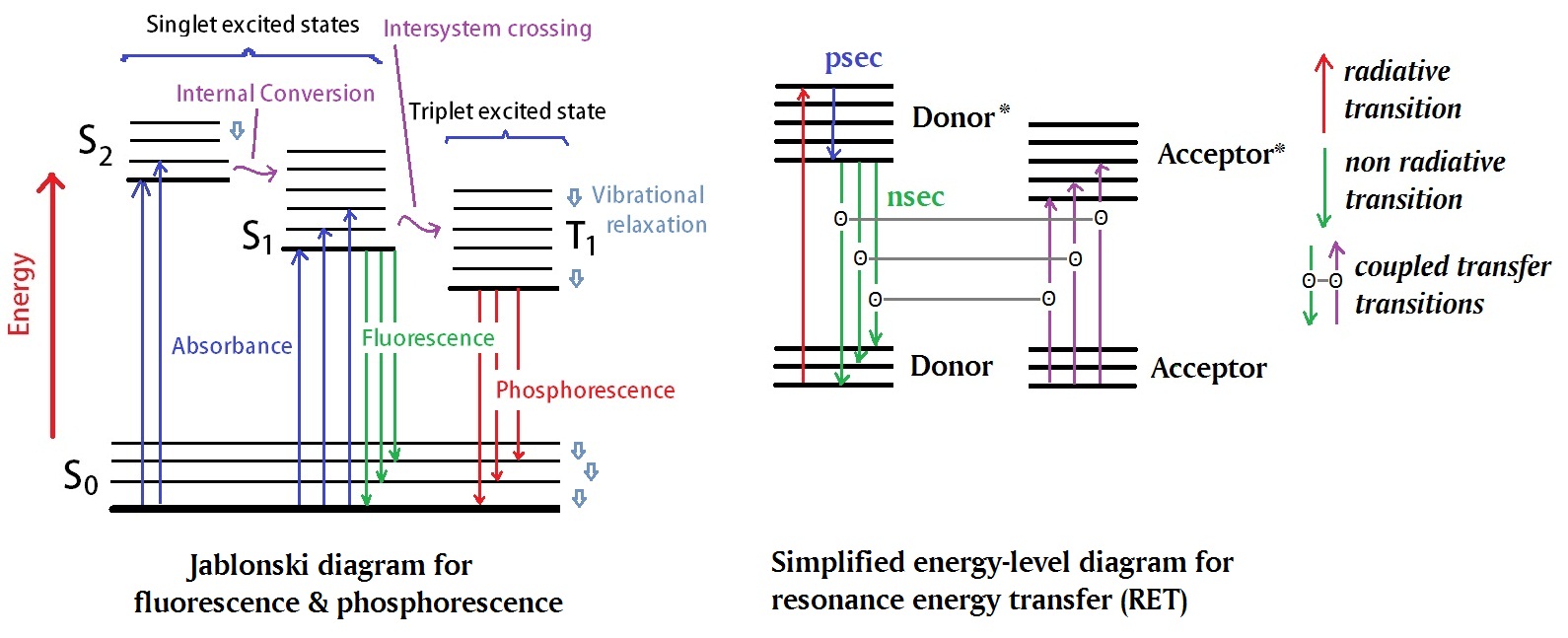
Figure 1: The classical Jablonski diagram, shown her in the left panel, illustrates electronic states of a molecule as well as photo induced processes related to absorption and emission of energy. A simplified energy-level diagram illustrating resonance energy transfer is shown on the right panel. An asterisks denotes an excited state.
What is fluorescence quenching?
Fluorescence quenching is any process that decreases the fluorescence intensity of a sample. Many molecular interactions can result in quenching including excited-state reactions, molecular rearrangements, energy transfer, ground-state complex formation, and collisional quenching. The efficiency of fluorescence quenching is distance dependent. If the reporter fluorophore and quencher are far apart, fluorescence occurs. However, if the reporter and quencher are close together in space fluorescence is suppressed and does not occur. In oligonucleotide probes, the reporter and quencher are typically placed such that a change in distance will produce a maximal change in fluorescence. The observed fluorescent signal monitors the event, for example, a hybridization or nuclease activity. In this case the oligonucleotide sequence acts as a flexible tether or link between the fluorescent reporter and quencher. Since, many dyes are known to aggregate, self-associate, form dimers, trimers, or polymers, the tendency for dyes to aggregate is the basis of the static quenching mechanism.
In static quenching or contact quenching a reporter such as FAM and a quencher such as BHQ-1 label can bind together to form a new, nonfluorescent intramolecular dimer. Furthermore, the efficiency of static quenching is dependent on the affinity of the reporter and quencher for each other. Often the reporter and quencher are planar, hydrophobic molecules that stack together to avoid contact with water.
Reference
dos Remedios CG, Moens PD.; Fluorescence resonance energy transfer spectroscopy is a reliable "ruler" for measuring structural changes in proteins. Dispelling the problem of the unknown orientation factor. J Struct Biol. 1995 Sep-Oct;115(2):175-85.
Choosing Reporter-Quencher Pairs for Efficient Quenching Through Formation of Intramolecular Dimers. Authors: Johansson, M.K. Book: Methods in Molecular Biology, v. 335; V.V. Didenko, Ed; Humana Press: Totowa, NJ, 2006; pp 17-29.
Intramolecular Dimers: A New Design Strategy for Fluorescence-Quenched Probes. Authors: Johansson, M.K.; Cook, R.M. Journal: Chem.-Eur. J. 2003, 9, 3466.
Intramolecular Dimers: A New Strategy to Fluorescence Quenching in Dual-Labeled Oligonucleotide Probes. Authors: Mary Katherine Johansson, Henk Fidder, Daren Dick and Ronald M. Cook. Journal: J. Am. Chem. Soc. 2002, 124, 6950.
Joseph R. Lakowicz; ; Principles of Fluorescence Spectroscopy. Editors: ISBN: 978-0-387-31278-1 (Print) 978-0-387-46312-4 (Online)
Efficiencies of fluorescence resonance energy transfer and contact-mediated quenching in oligonucleotide probes. Authors: Salvatore A. E. Marras, Fred Russell Kramer and Sanjay Tyagi. Journal: Nucleic Acids Research, 2002, 30, e122.
Peng L, Minbo H, Fang C, Xi L, Chaocan Z.;The interaction between cholesterol and human serum albumin. Protein Pept Lett. 2008;15(4):360-4.