Bioconjugates: Development and Applications of Nucleic Acids and Peptides Conjugated to Lipids and/or Carbohydrates
The availability of synthetic nucleic acids/oligonucleotides and peptides, areas where BioSynthesis has extensive experience, has promoted the development of conjugates of these molecules cross-linked to compounds such as lipids, carbohydrates and small molecules (drugs) to yield products with distinct properties. Taking advantage of its expertise in nucleic acids and peptide synthesis, BioSynthesis is developing new approaches for the production of different bioconjugates. From the point of view of therapeutic applications, because of their potential uses in genetic therapy and blocking gene expression the bioconjugates of nucleic acids are the most important ones. In general these bioconjugates acquire new properties, such as resistance to degradation and passage of the hydrophilic and anionic oligonucleotide across the hydrophobic negatively charged cell membrane or transduction. Yet, a suitable bioconjugate should maintain practically all of the oligonucleotide’s biological properties.
Lipid-oligonucleotide conjugates
The main objective of cross-linking a lipid moiety to an oligonucleotide, either an oligodeoxynucleotide (ODN) or oligoribonucleotide (ORN), is to increase the hydrophobic character of the latter and its lipid-solubility. This way a conjugate would pass across the highly lipophilic cell membrane and into the cytosol, a process called transduction. Yet, depending on the lipid’s nature these conjugates may have also some other new biological properties.
Cholesterol is a lipid that has been extensively used in the production of these conjugates. The cholesterol tag can be added at the 3’ or 5’ end of an ODN usually using a C4- to C8-linker or a polyethylene glycol linker. Cholesterol-ODN conjugates in addition to better transduction show improved nuclease resistance and anti-viral activity. In addition, these conjugates can form very stable duplex and triplex structures, with some small 3’-cholesterol modified ODN duplexes showing anti-tumor activity. Conjugation of antisense phosphorothioate ODN with cholesterol yields compounds with a higher antisense activity than their unconjugated counterparts. Cholesterol has been also used to produce conjugates of siRNA showing an improved cellular uptake. Because of its nature, siRNA when delivered as a complex with cationic lipids into the cell’s endosomes are recognized by TLR7 and TLR8, leading to the production of inflammatory cytokines. Replacement of the cationic lipids by covalently bound cholesterol does not stimulate such an immune response. Cholesterol can be replaced by plant-derived sterols, steroids and other related compounds (Fig 1). An advantage of using these products as tags is that frequently they target specific cell receptors, thus providing some degree of tissue specificity. Cholesterol and other natural and synthetic sterols can also be used to make conjugates of peptide nucleic acids or various nucleic acid analogs such as BNA.
In addition to cholesterol and other sterols, additional lipids such as alkyl chains, phospholipids, fatty acids and lipid substituted crown ethers can also be used (Fig 1). Like cholesterol-ODN conjugates, ODNs conjugated to alkyl chains larger than 12 carbons would bind to the serum lipoproteins LDL and HDL to form complexes that are taken up by these proteins’ cell receptors facilitating their entry. Lipophilic dendrimers have been conjugated to either the 5’- or 3’-ends of antisense ODNs to increase their cellular uptake. Increase in the size of the conjugate’s dendrimer results in a significant decrease in binding activity as shown by a marked drop in melting temperature.
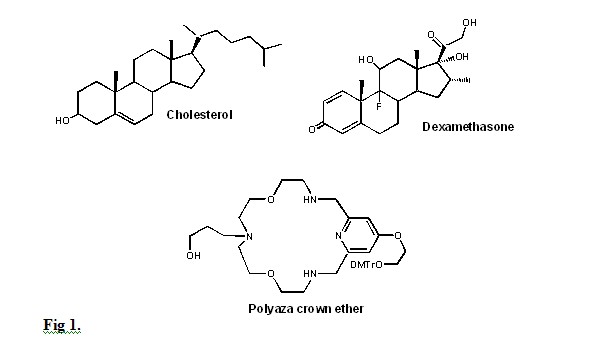
Another approach to ODN delivery is the use of ODN conjugates of polyethylene glycol (PEG) that has been applied to antisense ODN. PEG-ODN conjugates have a diblock copolymer-like structure where the ODN segment can interact with a cationic fusogenic peptide (rich in lysine and arginine, KALA) to form a polyelectrolyte core while the PEG segments are protruding from the micelle. These micelles are taken up by the cells via endocytosis to effectively deliver the antisense ODNs to their endosomal compartment. The KALA peptide associated to the ODN by disrupting the endosomal membrane allows the antisense ODN to enter the cell’s cytosol. The conjugation process Cross-linking of lipids to oligonucleotides can usually take place at either their carbohydrate moiety, deoxyribose or ribose, or their single terminal 5’ phosphate group. Cholesterol with a spacer can be obtained by reacting the cholesteryl-chloroformate with 6-amino-1-hexanol to yield a C6 spacer linked to cholesterol by an amide bond. However, the length of the spacer can be from 3 to 8 carbons. The terminal –OH of this spacer can then be phosphitylated with 2-cyanoethyl-N,N,N’,N’ tetraisopropyl phosphoramidite to yield a phosphoramidite that would react with the 5’-OH of the terminal deoxyribose (Fig. 2).
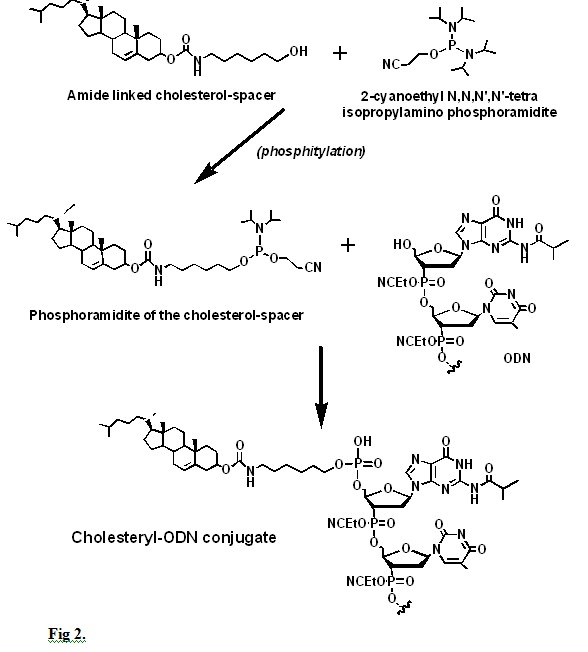
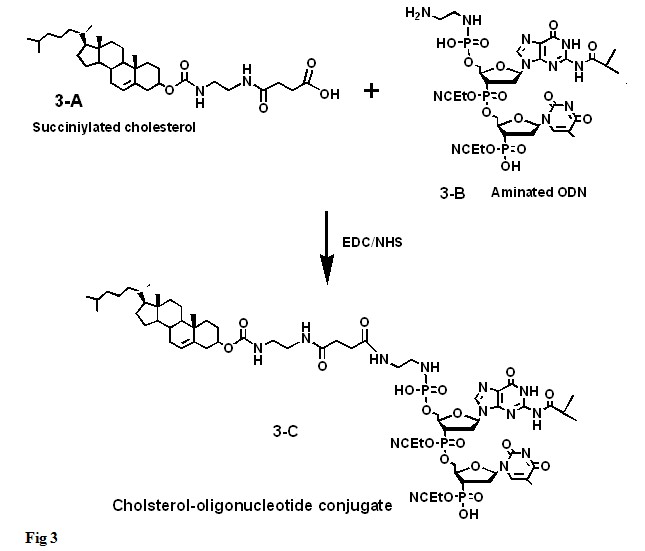
In ORNs where the 2’ –OH from the riboses are protected, the intermediate would react with the unprotected 5’ –OH. The oligonucleotide will be cross-linked to cholesterol via a spacer (Fig. 2). Cholesterol can also be added to the terminal 5’ phosphate of an oligonucleotide by reacting the cholesteryl-chloroformate with a diamine’s excess, e.g. ethylenediamine, to yield an amidated cholesteryl that is separated from the excess of diamine using silica gel chromatography. The amidated cholesteryl upon reaction with succinic anhydride would yield a larger side chain with a terminal carboxyl group (Fig. 3-A) that can be activated with a water-soluble carbodiimide (EDC) and N-hydroxysuccinimide (NHS). The activated cholesteryl derivative is them reacted with an oligonucleotide that has an amino alkyl group linked to the 5’ phosphate (Fig. 3-B) to yield a cholesterol oligonucleotide conjugate (Fig, 3-C).
Fatty acids, alkylamines, alkyl alcohols and other lipids can be also added following procedures similar to those described for cholesterol.
Applications
In addition to facilitate the transduction of oligonucleotides across cell membranes, another application of lipid-oligonucleotide conjugates is targeting of organs and specific cells. For instance, conjugation of an oligonucleotide and one cholesterol molecule to form a 3’-cholesterol-ODN results in a significant uptake by the liver. However, incorporation of another cholesterol to yield a 3’, 5’-bischolesteryl conjugate resulted in an almost quantitative uptake by the liver. An elegant example of the properties that steroids can confer to conjugates of DNA, RNA, BNA and others oligonucleotides analogs can be found in the peptide nucleic acid-steroid dexamethasone conjugates that bind to the cytoplasmic glucocorticoid receptor (GR). The complex formed by such kind of conjugate and the GR can then translocate from the cytoplasm into the nucleus.
Carbohydrate-oligonucleotide conjugates
These conjugates can have the carbohydrate moiety linked directly to the oligonucleotide or can be linked to a lipid or a peptide/protein. Due to the diversity of carbohydrate receptors present on the cell’s surface, oligosaccharides are useful for targeting conjugates to specific cells. For example, mannose linked to ODNs allows targeting of the mannose receptors present on certain cells and the same approach has been used for galactose. ODNs conjugated to sucrose show a better stability against enzymatic degradation. Sugar-ODN conjugates can form hybrid duplexes to complementary DNA with a higher affinity than that observed with natural DNA. Conjugation of antisense ODNs to a neoglycoprotein in which the protein has several mannose-6-phosphate residues, is readily internalized by cells having a receptor for this ligand on their surfaces. In contrast to peptides and lipids, the number of carbohydrate-oligonucleotide conjugates is relatively small. Because sugars cannot translocate the cell membranes the sugar residues are usually present in combination with either lipids or peptides to target the lectins present of the cell’s surface.