Instruction of reduction reaction using TCEP
Description of TCEP·HCl
TCEP•HCl, Tris(2-carboxyethyl)phosphine hydrochloride. Molecular Weight: 286.64; CAS #51805-45-9
Safety and Storage: Upon receipt store at room temperature in sealed container to prevent oxidation.
TCEP is a potent, versatile, non-volatile, odorless, thiol-free reducing agent with broad application in reduction of disulfide bonds (Figure 1). The unique compound is easily soluble and very stable in many aqueous solutions. The hydrochloride salt (TCEP•HCl, MW 286.64) has a solubility in water of 310 g/L (1.08 M). Being hydrophilic, TCEP is generally very soluble in aqueous buffers at nearly any pH. TCEP has only minimal solubility in organic solvents, including methanol and ethanol. TCEP is stable in aqueous, acidic, and basic solutions. Studies indicate that no change in concentration of TCEP occurs after 24 hour incubation at room temperature in 100 mM HCl, 100 mM NaOH, and most of 50 mM buffer system.

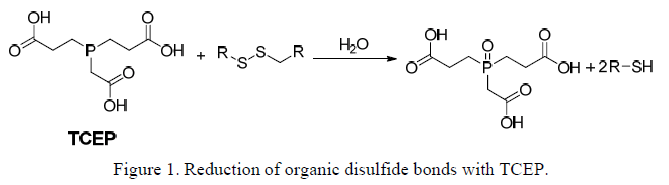
TCEP reduces disulfide bonds, for example in oligonucleotides, proteins and peptides, as effectively as dithiothreitol (DTT), but unlike DTT and other thiol-containing reducing agents, TCEP does not have to be removed before certain sulfhydryl-reactive cross-linking reactions. TCEP selectively and completely reduces even the most stable water-soluble alkyl disulfides over a wide pH range. TCEP effectively reduces disulfide bonds over a broad pH range. A pH range (1.5 <9.0) is recommended for the reduction reaction. Reductions frequently require less than 5 minutes at room temperature unlike most other reducing agents. TCEP is resistant to air oxidation. Compared to DTT, TCEP is more stable, more effective, and able to reduce disulfide bonds at lower pHs.
Procedure for the reduction reaction
Into a buffer solution containing oligos, 10 molar equivalents of TCEP was added under stirring at room temperature. The mixed solution was stirred at r.t. for about 10 minutes. The more TCEP used in the reaction, the faster the reduction reaction complete. Generally, S-S bonds on oligos could be reduced into SH groups.
Except the oligo-O-(CH2)3SH group, there is another chemical with thiol group, HS-(CH2)3OH, formed in this reducing method due to the structure of oligo-O-(CH2)3-S-S-(CH2)3OH provided. This chemical, HS-(CH2)3OH, should be considered before the next step reaction. For example, in the reaction involving maleimide, we recommended that more maleimide (2-fold of the regular amount) should be used.
Notes: TCEP is not particularly stable in phosphate buffers, especially at neutral pH. Therefore, if TCEP is to be used in PBS buffers, prepare the working solution immediately before use.
Contact us at BSI for all your needs for disulfide modified molecules, may they be oligonucleotides, modified oligonucleotides, peptides or proteins.