In cells, DNA damage leads to heritable mutations that can cause and accelerate disease progression and drug resistance in pathogenic bacteria as well as in cancers. However, all cells possess DNA-repair enzymes that can minimize the number of naturally occurring mutations. New imaging tools now allow monitoring real-time dynamics of mutagenesis to reveal the repair of DNA and the establishment of damage tolerance in cells.
The term “Mutagenesis” refers to a change in the genetic information of an organism resulting in a mutation. Genetic mutations can occur spontaneously or as a result of exposure to mutagens, agents that cause mutations. When mutations occur, the nucleotide sequence of a small genomic region is changed. The majority of mutations appear to be point mutations in which one nucleotide gets replaced with another. However, some mutations involve the insertion or a deletion of one or a few nucleotides. Also, errors in DNA replication or damaging effects of mutagens that change the structure of individual nucleotides can cause mutations. Chemicals such as bromouracil, or 5-bromouracil (5-Bru), induce DNA mutations that can be used as experimental mutagens for example for the study of repair mechanisms. 5-BrU causes point mutations via base substitution resulting in a base pair change from an A-T to a G-C or from a G-C to an A-T after for er of replication cycles. However, the formation of the exact base-pair depends on whether 5-BrU is positioned within the DNA molecule or is an incoming base when it is enolized or ionized.
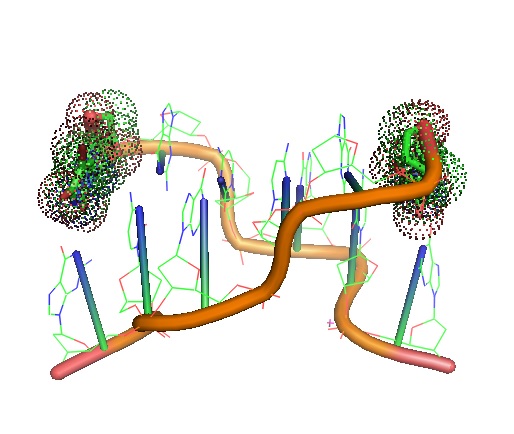
Figure 1: STRUCTURE OF A BROMOURACIL-GUANINE BASE PAIR MISMATCH IN A Z-DNA FRAGMENT. 5-BrU is a brominated derivative of uracil that acts as an antimetabolite or base analog. 5-BrU can be substituted for thymine in DNA where it can induce DNA mutation similarly as 2-aminopurine. It is often used as an experimental mutagen. https://www.rcsb.org/structure/1DA1
Mutagenesis can lead to cancer and various heritable diseases. However, some individuals with disease-causing mutations fail to show or express the disease phenotype. This phenomenon is known as ‘reduced or incomplete penetrance.’ Therefore not all ‘disease-causing’ mutations cause the specific disease. Therefore, reduced penetrance can be a function of the particular mutation(s) involved or of allele dosage. The newly emerged technology called ‘next-generation sequencing’ may shed more light on how genetic variants influence clinical phenotypes soon.
Genomes have a maintenance system that repairs, regulates and maintains DNA in response to DNA damage or drug treatments potentially causing lethal mutations. For decades, intensive research has been done to enhance our understanding of the molecular mechanisms of mutagenesis during healthy cell growth and in response to DNA damage.
In the last decades, many genes affecting mutation rates and regulatory mechanisms controlling their expression were identified. Despite this, we still lack a clear understanding of how replication and repair mechanisms as a whole define mutation rates. Furthermore, it also is not yet clear what determines what mutagenic DNA lesion is accurately getting repaired or converted into a mutation? Therefore it is still unclear which molecular machinery controls mutation rates, and despite extensive characterization of individual DNA repair and damage tolerance pathways, it is still unclear how they influence mutation rates.
Many chemicals naturally present or synthetically introduced to the environment can cause mutations. These mutagens can cause mutations in different ways. For example, base analogs are mistakenly used as substrates during the synthesis of new DNA in cells. Mutagens that react directly with the nucleotides in DNA cause structural changes. Other mutagens may not react directly with DNA but rather cause the cell to synthesize cell-damaging chemicals such as peroxides. However, the range of mutagens is so extensive that it is difficult to classify them all. Besides, radiation can also be a cause of mutations.
Modified nucleic acids, when incorporated into DNA or RNA, are useful tools for the study of mutagenesis as well as repair mechanisms and it machinery.
Reference
Cooper DN, Krawczak M, Polychronakos C, Tyler-Smith C, Kehrer-Sawatzki H. Where genotype is not predictive of phenotype: towards an understanding of the molecular basis of reduced penetrance in human inherited disease. Hum Genet. 2013;132(10):1077-130. doi: 10.1007/s00439-013-1331-2. Epub 2013 Jul 3. PubMed PMID: 23820649; PubMed Central PMCID: PMC3778950.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3778950/
https://www.ncbi.nlm.nih.gov/books/NBK21114/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC339574/
Stovall GM, Bedenbaugh RS, Singh S, Meyer AJ, Hatala PJ, Ellington AD, Hall B. In vitro selection using modified or unnatural nucleotides. Curr Protoc Nucleic Acid Chem. 2014 Mar 26;56:9.6.1-33. doi: 10.1002/0471142700.nc0906s56. PubMed PMID: 25606981; PubMed Central PMCID: PMC4068349. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4068349/
Uphoff S. Real-time dynamics of mutagenesis reveal the chronology of DNA repair and damage tolerance responses in single cells. Proc Natl Acad Sci U S A. 2018 Jul 10;115(28):E6516-E6525. doi: 10.1073/pnas.1801101115. Epub 2018 Jun 25. PubMed PMID: 29941584; PubMed Central PMCID: PMC6048535. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6048535/
---…---