|
|
|
Bio-Synthesis Newsletter - March-2016
|
Guanidinium-Rich Oligophosphoester For Drug and Probe Delivery
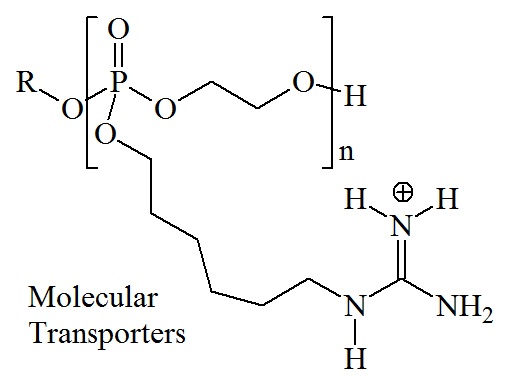 Recently a new type of cell-penetrating molecules has been designed. Researchers at Stanford University designed and synthesized guanidinium-rich oligophosphoesters and demonstrated that these molecules can penetrate cell to allow for the selective delivery of drugs and probes into cells. Guanidinium-rich oligophosphoesters appear to penetrate cell membranes in a similar manner as cell-penetrating peptides. Cell-penetrating peptides are short and usually arginine-rich peptides capable of transporting a wide range of biomolecules into living cells. Arginine-rich peptides have strong affinities for multiple negatively charged plasma groups. Highly positively charged molecules bind to fatty acids in the cell membrane from which they are released into the cytosol. Guanidinium-rich oligophosphoesters are a new addition to the repertoire of oligomeric scaffolds useful for the design and synthesis of a variety of cell-penetrating molecules. Recently a new type of cell-penetrating molecules has been designed. Researchers at Stanford University designed and synthesized guanidinium-rich oligophosphoesters and demonstrated that these molecules can penetrate cell to allow for the selective delivery of drugs and probes into cells. Guanidinium-rich oligophosphoesters appear to penetrate cell membranes in a similar manner as cell-penetrating peptides. Cell-penetrating peptides are short and usually arginine-rich peptides capable of transporting a wide range of biomolecules into living cells. Arginine-rich peptides have strong affinities for multiple negatively charged plasma groups. Highly positively charged molecules bind to fatty acids in the cell membrane from which they are released into the cytosol. Guanidinium-rich oligophosphoesters are a new addition to the repertoire of oligomeric scaffolds useful for the design and synthesis of a variety of cell-penetrating molecules.
|
|
Read More
|
|
|
mRNA localization signals act as zipcodes for RNA localization
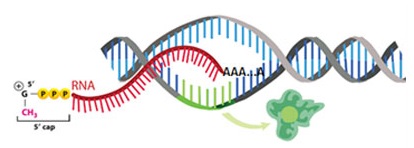 In cells newly synthesized proteins are targeted to their subcellular location in eukaryotic organisms, often via signal peptide sequences. In addition to signal polypeptide sequences within proteins, mRNA also contains sequence information for their selective localization to particular cytoplasmic sites. mRNA localization signals, or zipcodes, are formed by discrete regions, often in the 3’UTR, in mRNA. These signals bind to a series of specific proteins that form ribonucleoprotein (RNP) localization complexes. It appears that the composition of these complexes determines the transport, sorting, and localization of transcripts in the cytoplasm. Synthetic mRNAs, including modified mRNAs, can be used as tools for the study of sorting, transport and localization pathways involving mRNA. Unmodified and modified mRNAs are automatically synthesized as useful biochemical tools for molecular biology. In cells newly synthesized proteins are targeted to their subcellular location in eukaryotic organisms, often via signal peptide sequences. In addition to signal polypeptide sequences within proteins, mRNA also contains sequence information for their selective localization to particular cytoplasmic sites. mRNA localization signals, or zipcodes, are formed by discrete regions, often in the 3’UTR, in mRNA. These signals bind to a series of specific proteins that form ribonucleoprotein (RNP) localization complexes. It appears that the composition of these complexes determines the transport, sorting, and localization of transcripts in the cytoplasm. Synthetic mRNAs, including modified mRNAs, can be used as tools for the study of sorting, transport and localization pathways involving mRNA. Unmodified and modified mRNAs are automatically synthesized as useful biochemical tools for molecular biology.
|
|
Read More
|
|
|
Glycosylated Peptides
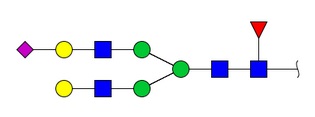 Glycosylation is a post-translational modification present on specific amino acid residues in glycoproteins and glycopeptides. In higher animals, many genes are expressed as glycoproteins. In the glycoprotein, a specific polypeptide sequence is associated with a number of covalently attached oligosaccharide chains. The attachment of a carbohydrate moiety to asparagine is a very common post-translational modification, known as an N-linked glycopeptide. Another common attachment is the linkage of a carbohydrate moiety to serine or threonine residues, known as O-linked glycopeptides. Changes in the glycosylation pattern of physiological glycopeptides are known to cause non-desirable physiological effects such as acute inflammation, accelerated aging, diabetes mellitus, and allergies, among others. Many glycan structures are known, and structural databases of these are available. The presence of glycosyl moieties on a peptide or protein can improve its biological activity, increase half-live, enhance solubility, and improve therapeutic response and tolerance for peptide-based drugs. Glycosylation is a post-translational modification present on specific amino acid residues in glycoproteins and glycopeptides. In higher animals, many genes are expressed as glycoproteins. In the glycoprotein, a specific polypeptide sequence is associated with a number of covalently attached oligosaccharide chains. The attachment of a carbohydrate moiety to asparagine is a very common post-translational modification, known as an N-linked glycopeptide. Another common attachment is the linkage of a carbohydrate moiety to serine or threonine residues, known as O-linked glycopeptides. Changes in the glycosylation pattern of physiological glycopeptides are known to cause non-desirable physiological effects such as acute inflammation, accelerated aging, diabetes mellitus, and allergies, among others. Many glycan structures are known, and structural databases of these are available. The presence of glycosyl moieties on a peptide or protein can improve its biological activity, increase half-live, enhance solubility, and improve therapeutic response and tolerance for peptide-based drugs.
|
|
Read More
|
|
|
Glycosylated Oligonucleotides or Oligonucleotide Conjugates
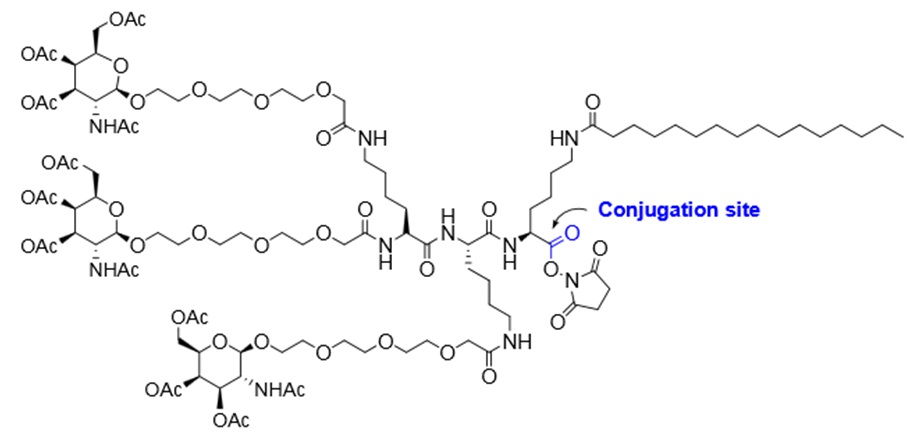 Oligonucleotide-carbohydrate conjugates can be used in antisense technologies as well as for functional studies of glycosylated DNA. The covalent attachment of carbohydrate moieties to oligonucleotides influences their biodistribution and cellular uptake. Sugar-macromolecule conjugates are useful as selective delivery systems and are potential candidates for the targeted delivery of antisense oligonucleotides or siRNA compounds. For example, N-acetylgalactosamine (GalNAc) conjugates have been used for subcutaneous applications and are known to increase bioavailability in hepatocytes. Also, triantennary GalNac conjugates are useful as delivery systems in siRNA and antisense technology. A liver-targeted siRNA conjugate composed of chemically stabilized siRNA and a trivalent GalNac ligand has already shown promise in the disease treatment. In this type of conjugate, the 3’terminus of a siRNA sense strand is attached to three GalNac molecules targeting asialoglycoprotein receptor 1 (ASGPR) on hepatocytes. Synthesis of oligonucleotide-carbohydrate conjugates is possible using a combination of solid-phase synthesis and post-synthesis coupling chemistry. Oligonucleotide-carbohydrate conjugates can be used in antisense technologies as well as for functional studies of glycosylated DNA. The covalent attachment of carbohydrate moieties to oligonucleotides influences their biodistribution and cellular uptake. Sugar-macromolecule conjugates are useful as selective delivery systems and are potential candidates for the targeted delivery of antisense oligonucleotides or siRNA compounds. For example, N-acetylgalactosamine (GalNAc) conjugates have been used for subcutaneous applications and are known to increase bioavailability in hepatocytes. Also, triantennary GalNac conjugates are useful as delivery systems in siRNA and antisense technology. A liver-targeted siRNA conjugate composed of chemically stabilized siRNA and a trivalent GalNac ligand has already shown promise in the disease treatment. In this type of conjugate, the 3’terminus of a siRNA sense strand is attached to three GalNac molecules targeting asialoglycoprotein receptor 1 (ASGPR) on hepatocytes. Synthesis of oligonucleotide-carbohydrate conjugates is possible using a combination of solid-phase synthesis and post-synthesis coupling chemistry.
|
|
Read More
|
|
|
|
|
|
|
Bio-Synthesis, Inc.
800 Mario Court, Lewisville, TX 75057, USA
Toll Free: 800.227.0627 | 1.972.420.8505 (Intl.)
|
|
|
|
|