|
|
|
Bio-Synthesis Newsletter - March 2019
|
Splice switching BNAs
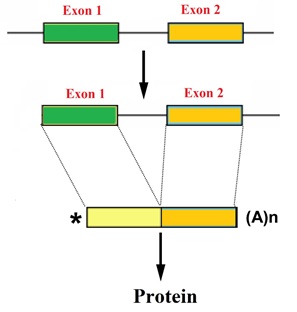 Oligonucleotides modified with BNAs allow the design of splice-switching oligonucleotide (SSO) probes. Oligonucleotides modified with BNAs allow the design of splice-switching oligonucleotide (SSO) probes.
Short, synthetic, oligonucleotides modified with BNAs can act as antisense molecules. BNA oligonucleotides base-pair with pre-mRNA with high affinity and disrupt normal splicing of transcripts by blocking the RNA–RNA base-pairing or protein–RNA binding interactions that occur between components of the splicing machinery and pre-mRNAs.
Exon skipping splice-switching oligonucleotides can be designed rationally by selecting an antisense sequence targeting the exon that is supposed to be skipped. Design parameters to consider include the activity of the splice-switching oligonucleotide, the melting temperature, the guanine-cytosine content, the length of the oligonucleotide, as well as the secondary structure or sequence motif corresponding to a splicing signal of the target RNA that influence the activity of the splice-switching oligonucleotide.
|
|
Read More
|
|
|
Genomic Labeling with CRISPR/Cas9
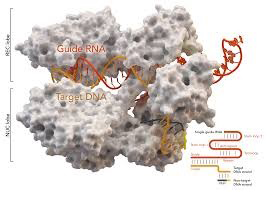 A fast, new method utilizing CRISPR/Cas9 enables labeling of genomic sequences in various species. Ishii et al . reported in 2019 the development of a method for the visualization of genomic spatiotemporal organization. The research group believes that this technique will increase our understanding of how chromatin structure and function are intertwined. The method was as a tool for the visualization of defined genomic sequences in fixed nuclei and chromosomes based on a two‐part guide RNA with a recombinant Cas9 endonuclease complex. Unlike classical fluorescence in situ hybridization, this new method does not require DNA denaturation and therefore permits better structural chromatin preservation. Furthermore, using differentially labeled trans‐activating crRNAs also enable multiplexing. Furthermore, a CRISPR/Cas9‐mediated DNA labeling process allows for kinetic measurements in real-time. A fast, new method utilizing CRISPR/Cas9 enables labeling of genomic sequences in various species. Ishii et al . reported in 2019 the development of a method for the visualization of genomic spatiotemporal organization. The research group believes that this technique will increase our understanding of how chromatin structure and function are intertwined. The method was as a tool for the visualization of defined genomic sequences in fixed nuclei and chromosomes based on a two‐part guide RNA with a recombinant Cas9 endonuclease complex. Unlike classical fluorescence in situ hybridization, this new method does not require DNA denaturation and therefore permits better structural chromatin preservation. Furthermore, using differentially labeled trans‐activating crRNAs also enable multiplexing. Furthermore, a CRISPR/Cas9‐mediated DNA labeling process allows for kinetic measurements in real-time.
|
|
Read More
|
|
|
Viruses outsmart their host cells
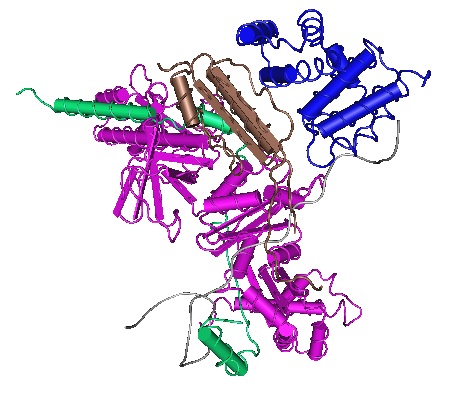 Viruses need a host cell to replicate. Even though viruses can spread by surviving outside a host, they need a host for replication because they lack the complex apparatus necessary for the transcription of genetic information and its subsequent translation into new virus components. But how does a virus induce its host to transcribe its genetic information to produce daughter viruses? With the help of high-resolution cryo-electron microscopy researchers have now successfully deciphered this process. Viruses need a host cell to replicate. Even though viruses can spread by surviving outside a host, they need a host for replication because they lack the complex apparatus necessary for the transcription of genetic information and its subsequent translation into new virus components. But how does a virus induce its host to transcribe its genetic information to produce daughter viruses? With the help of high-resolution cryo-electron microscopy researchers have now successfully deciphered this process.
A virus prevents the RNA polymerase from terminating the transcription process. A small protein called 'lambda-N' (λN) attaches itself to the host's RNA polymerase. The resulting complex forces the host polymerase to continue transcription of the viral genes. Because of the stability of the so formed protein complex, the RNA polymerase continues transcribing even once it reaches the viral genes.
|
|
Read More
|
|
|
Tau clearance in brains
 In Alzheimer’s disease and other disease involving the cytoskeletal protein tau called “tauopathies,” the spreading of seeds of the protein TAU is through to cause the disease affecting abnormal tau species. The accumulation of insoluble tau aggregates may be the cause of progressive neurodegeneration. The recently discovered lymphatic system of the brain, residing within the dura of the meninges, plays an essential role in draining macromolecules from the brain to the cerebrospinal fluid. The injection of microRNA-labeled TAU into mice brains revealed that wild type mice cleared TAU faster than transgenic mice lacking dural lymphatics. The use of a near-infrared (NIR) imaging technique together with fluorescence molecular tomography (FMT) allowed the investigation of the removal of extracellular TAU by the lymphatic system in a mouse model. The research showed that the brain’s lymphatic system contributed to TAU clearance in the mice. However, additional data indicated that the lymphatic pathway does not exclusively mediate TAU clearance. Hence TAU covalently linked to oligonucleotides, in particular to miRNAs may offer themselves as tools for the study of taupathies on the molecular level. In Alzheimer’s disease and other disease involving the cytoskeletal protein tau called “tauopathies,” the spreading of seeds of the protein TAU is through to cause the disease affecting abnormal tau species. The accumulation of insoluble tau aggregates may be the cause of progressive neurodegeneration. The recently discovered lymphatic system of the brain, residing within the dura of the meninges, plays an essential role in draining macromolecules from the brain to the cerebrospinal fluid. The injection of microRNA-labeled TAU into mice brains revealed that wild type mice cleared TAU faster than transgenic mice lacking dural lymphatics. The use of a near-infrared (NIR) imaging technique together with fluorescence molecular tomography (FMT) allowed the investigation of the removal of extracellular TAU by the lymphatic system in a mouse model. The research showed that the brain’s lymphatic system contributed to TAU clearance in the mice. However, additional data indicated that the lymphatic pathway does not exclusively mediate TAU clearance. Hence TAU covalently linked to oligonucleotides, in particular to miRNAs may offer themselves as tools for the study of taupathies on the molecular level.
|
|
Read More
|
|
|
|
|
Bio-Synthesis, Inc.
800 Mario Court, Lewisville, TX 75057, USA
Toll Free: 800.227.0627 | 1.972.420.8505 (Intl.)
|
|
|
|
|
|