Advantages:
- Superior Technical Support: Assist client from design to delivery
- High success rate: Bio-Synthesis's success rate for custom bioconjugates is more than 95%, higher than our competitors.
- Full Spectrum of Modification and Activation Services: Modification and derivatization of small molecules, biopolymers, small molecules, as well as creation of functionalized surfaces for immobilization and cross-linking.
- Flexible Technologies: Employ solid/liquid phase and microwave technologies for synthesis and modifications of functional target molecules.
- Optimized Chemistries: Expertise with hundreds of cross linking modification, activation and/or conjugation techniques using reactive cross linkers
- Guaranteed Quality: All samples are carefully monitored for stability and characterized to ensure batch to batch consistency.
Service Portfolios
- Azide and alkyne modification for Click Chemistry application
- Modification of an alcohol in proteins, carbohydrates, and user supplied compounds
- Modification with prodrug linkers
- Aldehyde and ketone addition
- Derivatization of carboxylic acids and carboxamides
- Attachment of single- or branched-chain polyethylene glycol (PEG) groups
- Protein sulfhydryl capping for certain enzymes for specific assays
- Conversion of amines to sulfhydryl group for sulfhydryl-specific cross-linkers and immobilization chemistries
- Conversion of sulfhydryl groups to carboxyl groups for peptide-protein conjugates
- Reversibly blocking of primary amines through the citraconic anhydride reaction
- Temporarily blocking of amine groups and extension of lysine side chains
- Irreversible blocking of primary amines
- Permanent blocking of sulfhydryl groups
- Reversible blocking of cysteines and other sulfhydryl groups.
- Capping of primary amines by addition of latent carboxyl groups.
- Oxidization of sugars into amine-reactive aldehyde groups
- Rapid carboxymethylation of reduced cysteine residues.
- Introduction of maleimide groups
- Disulfide Reduction
- Thiol Introduction through Trauts reagent or SATA
- Fab Preparation
- Modification with synthetic polymers: polyethylene glycol (PEG) and dextran
- Addition of cross linker, spacers
Standard Biomolecule Modification Service: BIOCON10000
Price:
The prices varies depending on the project specifications. The list price is for reference only. Our service includes materials and labor for conjugation only! Price does not include the cost of biopolymer synthesis and, if deemed necessary, biopolymer modification to introduce additional functional groups, extra linkers, and/or spacers. Please contact us for a quote.
Chemistry:
Coupling of preactivated small molecule and biomolecule with chemical reactive groups such as amine, thiol, carboxylate, hydroxyl, aldehyde and ketone, active hydrogen, photo-chemical and cycloaddition reactions, as well as zero-length cross-linking, homobifunctional, heterobifunctional or multifunctional cross-linking chemistries, dendrimer and dendrons and cleavable regent systems.
Some commonly used cross-linking reagents include:
- Glutaraldehyde - links carrier molecules to N-terminus of peptide
- Carbodiimide (EDC) - attaches carrier to C-terminus of peptide
- Succinimide esters (e.g. MBS, SMCC) - binds free amino group and Cys residues
- Benzidine (BDB) - links to Tyr residues
- Periodate - attaches to carbohydrate groups
- Bioconjugate chemistry may occur through the C- and N-terminals of each polypeptide chain, the carboxylate groups of aspartic and glutamic acids, the ϵ-amine of lysine, the guanidino group of arginine, the sulfhydryl group of cysteine, the phenolate ring of tyrosine, the indol ring of tryptophan, the thioether of methionine and the imidazole ring of histidine.
- Prodrug linkers: Peptide prodrug linkers, disulfide prodrug linkers, copper free Click Chemistry; Click Chemistry, thiol reactives, oxime/aldehyde chemistry
Service Specification:
After standard desalting or purification, a small percent of heterogeneous products containing single or multi-site conjugate per molecule may exist.
Material:
- Biopolymers
- Monomers: Amino acids, nucleotides, sugars
- Protein: Enzymes, antibodies, antigens, cell adhesion molecules
- Peptides: Synthetic polypeptides
- Saccharides: Sugars, oligosaccharides and polysaccharides
- Lipids: Fatty acids, phospholipids, glycolipids and any fat-like substances
- Ligands: Hormone receptors, cell surface receptors, avidin and biotin, small molecules
- Labels: Fluorescent dyes, infrared-absorbing and UV-Vis absorption chromophores, nonradioactive labels
- Nucleic acids and nucleotides: DNA, RNA, BNA, nucleic acid analogs and genomic DNA
- Synthetic polymers: PEG, Nanoparticles, gold particles, dendrimers, dendron, PAMAM
- Others: Conjugated or mixtures of any the above
- Solid Supports: Agarose, glass plates, membrane, beads
Procedure:
All custom synthesis of biomolecules, modifications or bioconjugation services are manufactured under strict quality control processes. Analytical HPLC and MS analyses are performed in every development cycle. Final target conjugates must first be isolated from excess or unreacted reagents. In many cases, simple dialysis may suffice to remove unreacted reagent from the reaction solution. Depending on the project scope, size-exclusion chromatography (SEC) or HPLC may also be used to either remove excess reagent or to isolate and characterize the cross-linked product. The Cross-linked target molecule may then be further characterized by biochemical or biophysical techniques. Once the product has been purified, it may be subject to various characterization methods including spectrometric (MALDI-TOF, ESI, LC-MS), fluorescence, electrophoresis, immunochemical, biochemical and enzymatic analysis.
QC (quality control) and QA (quality assurance) procedures are also
followed independently to ensure the highest quality possible of every delivered conjugate. Moreover, our dedicated technical account managers will guide you
through every step of the process and keep you informed of the latest project progress.
Delivery Specifications:
The typical delivery consists of lyophilized sample in individual, fully labeled vials. The shipment also contains COA, MS,
HPLC and/or other analytical data. Additional analytical data is also available upon request.
Bio-Synthesis can assist customers who want to introduce functional groups or modify their existing biopolymers such as native proteins, oligos, peptides or commercially available synthetic compounds, to be ready for conjugation with other biopolymers.
Sample Requirements
Non-commercial small molecule supplied by customer
User-supplied, non-commercial small or macromolecules should be sufficiently pure (≥95% pure). Please provide the QC data (typically HPLC, MS, ESI or NMR data etc.) and MSDS (if any) along with your compound. We can assist you with the purification and acquisition of analytical data. These compounds must contain functional groups that can be targeted for crosslinking including carboxylate groups, primary amine groups, aldehyde/keto residues, hydroxyl, hydrazine, hydrazide, aminoxy, saccharide /glycan groups or thiol reactive functional group(s). Coupling also can be nonselective using a photoreactive phenyl azide cross inker. If necessary, we can also assist you with the creation and activation of specific functionalities. Any sample supplied by the client requires a quality check by our analytical team to ensure sample quality prior conjugation.
Commercially available small molecules
Commercially available small molecules can be supplied by customers or ordered through Bio-Synthesis. Common small molecules such as common fluorescent dyes, biotin-NHS and other hetero-bifunctional cross-linkers are available at Bio-Synthesis. For in stock compounds, Bio-Synthesis will charge customers only the amount needed for conjugation. If the small molecules are to be purchased separately, the cost of acquiring this material will be added to the invoice along with a $50 administration charge per order.
Non-commercially available small molecules
We can custom synthesize small molecules in-house. A quotation will be prepared for such syntheses.
Oligonucleotide, oligomimetics and custom gene or genomic DNA
DNA, RNA and bridged nucleic acid analogs such as BNA can be synthesized in-house at Bio-Synthesis, followed by subsequent conjugation through amino, thiol, aldehyde, azide, carboxyl or 5'-phosphate modification. Oligos supplied by the customer should be HPLC or gel purified (>90% pure). Please provide the QC data needed for purity assessment. Extra charges may apply for analyzing the starting materials at Bio-Synthesis.
Peptides, peptidomimetics, and expressed protein
Peptides or proteins can be synthesized or expressed in-house at Bio-Synthesis. We label or conjugate peptides through N-terminal, C-terminal or internal side chain of an amino acids. Peptides supplied by the customer should be HPLC purified (>90% pure) and should contain a single modification site. We can make any standard peptides with functional groups incorporated through peptide synthesis. Please provide the peptide sequence of the desired peptide on the order form.
Antibodies
Commercial antibodies can be supplied by customers or ordered through Bio-Synthesis with an additional fee plus the cost of the antibody. Non-commercial biopolymers supplied by customers should be affinity purified. Please provide gel electrophoresis data along with your antibody. Bio-Synthesis also assists customers with antibody production, purification, modification and fragmentation prior to any cross linking reaction.
Chemistry of Reactive Group used
Every chemical modification or conjugation process involves the reaction of one functional group with another, resulting in the formation of a covalent bond. The creation of bioconjugate reagents with spontaneously reactive or selectively reactive functional groups, forms the basis for simple and reproducible crosslinking or tagging of target molecules. Our well-trained chemists assist clients from project scope collections to designs and determining appropriate homobifunctional or heterobifunctional cross-linking chemistries. We have delivered thousands of custom conjugated biopolymers and are fully capable of meeting the ever-increasing bioconjugation needs in biological and drug discovery research. Hundreds of reaction systems have applied in our organic laboratories.
- Amine Reactions: NHS ester, imidoester, hydroxymethyl phosphine, guanidination of amine, fluorophenyl esters, carbodiimides, anhydrides, arylating agents, carbonates, aldehydes, and glyoxals
- Thiol Reactions: Maleimide, Haloacetyl, Pyridyldisulfide, Vinyl sulfone, Thiol-disulfide exchange
- Carboxylate Reactions: Carbodiimides
- Hydroxyl Reactions: Isocyanates, enzymatic oxidation, Carbonyldiimidazole
- Aldehyde and Ketone Reactions: Hydrazine derivative, Schiff Base formation, reductive amination
- Active Hydrogen Reactions: Iodination reaction
- Photo-chemical Reactions: Psoralen compounds, aryl azides and halogenated aryl azides, bensophenones, anthraquinones
- Cycloaddition Reactions: Chemoselective ligation such as Click Chemistry, Diels-Alder reaction
Bio-Synthesis is committed to Total Quality Management (TQM) to assure customers' complete satisfaction. MS and HPLC analyses are performed following the completion of biopolymer synthesis, purification and QC (quality control). QA (quality assurance) procedures are also followed independently to double guarantee the high quality of every delivered peptide. Bio-Synthesis has Total Quality Management System (TQM) successfully upgraded to fully comply with ISO 9001:2008 regulations.
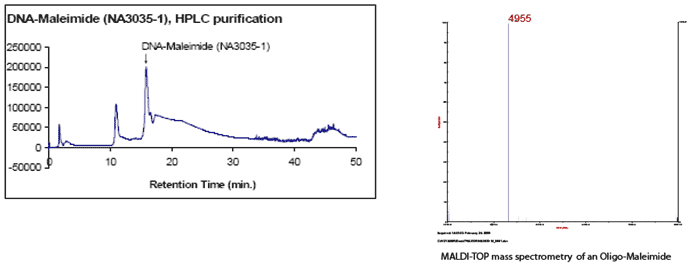
Case Study:
Sequence: TGT GTT CTA TGT CTT-Maleimide
MW: 4955.50
MS and HPLC results:
Ordering and Submitting Requests for Bioconjugation Services
For us to better understand your customized project, please complete our Bioconjugation Service Questionnaire. The more our chemists understand your project’s needs, the more accurate your provided feedback will be. Providing us with your project’s details enables us to recommend the best reagents to use for your project. The most useful and readily available tools for bioconjugation projects are cross-linking reagents. A large number of cross-linkers, also known as bifunctional reagents, have been developed. There are several ways to classify the cross-linkers, such as the type of reactive group, hydrophobicity or hydrophilicity and the length of the spacer between reactive groups. Other factors to consider are whether the two reactive groups are the same or different (i.e. heterobifunctional or homobifunctional reagents), spacer is cleavable and if reagents are membrane permeable or impermeable. The most accessible and abundant reactive groups in proteins are the ϵ-amino groups of lysine. Therefore, a large number of the most common cross-linkers are amino selective reagents, such as imidoesters, sulfo-N-hydroxysuccinimide esters and N-hydroxysuccinimide esters. Due to the high reactivity of the thiol group with N-ethylmaleimide, iodoacetate and a-halocarbonyl compounds, new cross-linkers have been developed containing maleimide and a-carbonyl moieties. Usually, N-alkylmaleimides are more stable than their N-aryl counterparts.
In addition to the reactive groups on the cross-linkers, a wide variety of connectors and spacer arms have also been developed. The nature and length of the spacer arm play an important role in the functionality. Longer spacer arms are generally more effective when coupling large proteins or those with sterically protected reactive side-chains. Other important considerations are the hydrophobicity, hydrophilicity and the conformational flexibility. Long aliphatic chains generally fold on themselves when in an aqueous environment, making the actual distance spanned by such linker arms less than expected. Instead, spacers containing more rigid structures (for example, aromatic groups or cycloalkanes) should be used. These structures, however, tend to be very hydrophobic which could significantly decrease the solubility of the modified molecules or even modify some of their properties. In such cases, it is recommended to choose a spacer that contains an alkyl ether (PEO) chain. Bio-Synthesis offers several cross-linkers with PEO chains, such as thiol-binding homobifunctional reagents, heterobifunctional bases and their derivatives.
Within 3-5 days upon receiving your project scope, we will provide you an appropriate quotation. An order can be placed with PO (Purchase Order) or major credit cards (  ). Your credit card will be billed under Bio-Synthesis, Inc.
). Your credit card will be billed under Bio-Synthesis, Inc.