5' Trimethoxystilbene Cap [5-TriCAP]
5' Trimethoxystilbene end cap, a chain terminator, can be use for covalently bridging the termini of oligonucleotide hairpins. In microarray experiments the stilbene-cap was shown to lead to a higher signal intensity and a better discrimination particularly of terminal mismatches.
Trimethoxystilbene is available as 5'-end modification; this modifier can be introduce at the time of synthesis.
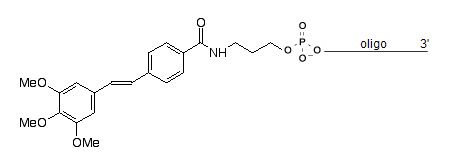 5'-Trimethoxystilbene, [ 5-TriCAP] Modification
5'-Trimethoxystilbene, [ 5-TriCAP] Modification
- 250 nmol: $250
- 1 umole: $350
- 5 umoles: $600
- 10 umoles: $850
Reference (s):
- Dogan, Z.; Paulini, R.; Rojas Stütz, J. A.; Narayanan, S.; Richert, C. 5’-Tethered stilbene derivatives as fidelity- and affinity-enhancing modulators of DNA duplex stability. J. Am. Chem. Soc. 2004, 126, 4762-4763.
- Narayanan, S.; Gall, J.; Richert, C. Clamping down on weak terminal base pairs: oligonucleotides with molecular caps as fidelity-enhancing elements at the 5’- and 3’-terminal residues. Nucleic Acids Res. 2004, 32, 2901-2911.
- Tuma, J.; Paulini, R.; Rojas Stütz, J. A.; Richert, C. How much pi-stacking do DNA termini seek? Solution structure of a self-complementary DNA hexamer with trimethoxystilbenes capping the terminal base pairs. Biochemistry 2004, 43, 15680-15687
- Dombi, K. L.; Griesang, N.; Richert, C. Oligonucleotide arrays from aldehyde-bearing glass with coated background. Synthesis 2002, 816-824.
5' Pyrene Cap
5' Pyrene end cap, also known as pyrenylmethylpyrrolindol, when introduce at 5' end of an oligo, will produce a cap that is more lipophilic than the trimethoxystilbene. The aromatic stacking moiety is linked to the terminus of the DNA through a more rigid, cyclic linker than in the case of 1This feature may prove advantageous for researchers interested in exploiting the special photophysical properties of the pyrenyl substituent. The pyrrolindol linker is stereoregular, leading to a single product that can be readily purified by HPLC. The pyrenyl cap is the lead compound discovered in a recent combinatorial study that evaluated over 40 different caps.2 The cap proved particularly successful for hybridization probes with a 5’-terminal deoxyadenosine residue.2 Again, its duplex-stabilizing effect does not require blunt ends. The tertiary amino group can be expected to be protonated at physiological pH, producing a cationic functionality that may help to attract target strands electrostatically. The five membered ring presenting the pyrenyl stacking unit mimics the deoxyribose of natural nucleosides, making duplexes terminating in this cap more similar in shape to unmodified DNA than those capped with the trimethoxystilbene.
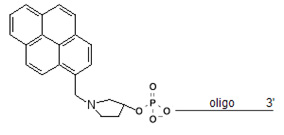
Pyrene cap is available as 5'-end modification, this modifier can be introduce at the time of synthesis.
5'-Pyrene Cap Modifiction
- 250 nmol: $250
- 1 umole: $350
- 5 umoles: $600
- 10 umoles: $850
Reference (s):
- Dogan, Z.; Paulini, R.; Rojas Stütz, J. A.; Narayanan, S.; Richert, C. 5’-Tethered stilbene derivatives as fidelity- and affinity-enhancing modulators of DNA duplex stability. J. Am. Chem. Soc. 2004, 126, 4762-4763.
- Narayanan, S.; Gall, J.; Richert, C. Clamping down on weak terminal base pairs: oligonucleotides with molecular caps as fidelity-enhancing elements at the 5’- and 3’-terminal residues. Nucleic Acids Res. 2004, 32, 2901-2911.
5'-Uaq Cap
3' Uaq cap can be introduced during synthesis at 3' end. Caps are covalently appended residues that stabilize double helices by binding to termini.1-3 Caps increase duplex melting points and improve base pairing fidelity by selectively binding to Watson-Crick base pairs. For short hybrid duplexes between DNA probes and RNA target strands, the increase is up to 18 °C.2 Further, appending the cap leads to larger DTms between duplexes of perfectly matched sequence and duplexes with a terminal mismatch, thereby improving base pairing fidelity at its terminus.
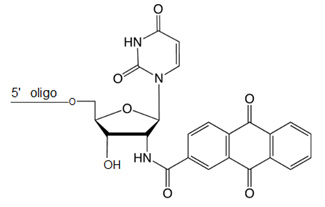
5' Uaq Cap Modification
- 250 nmol: $180
- 1 umole: $250
- 5 umoles: $350
- 10 umoles: $650
Reference (s):
- 5'-Trimethoxystilbene cap phosphoramidite, Glen Research catalog no. 10-1986, and 5'-pyrene cap phosphoramidite, Glen Research catalog no. 10-1987.
- A. Patra, C. Richert, High fidelity base pairing at the 3'-terminus. J. Amer. Chem. Soc. 2009, 131, 12671-12681.
- Tuma, J.; Paulini, R.; Rojas Stütz, J. A.; Richert, C. How much pi-stacking do DNA termini seek? Solution structure of a self-complementary DNA hexamer with trimethoxystilbenes capping the terminal base pairs. Biochemistry 2004, 43, 15680-15687.
Triphosphate Oligo Modification
Triphosphate modification of DNA or RNA oligonucleotides have become crucial biological importance in biochemical and therapeutic applications. They are antiviral 1 and anticancer 2 inhibitors, are substrates for polymerase chain reactions, nucleic acids ligation reactions, 4-6 are useful for structural and mechanistic studies, 7 results in immune response stimulation, 8 and are intermediates in the enzymatic synthesis of m7G-5'-ppp capped RNAs9-12 this is common for the maturation of mRNA. N7mGppp RNA capping is also available from Bio-Synthesis.
Chemical synthesis of triphosphorylated DNA or RNA oligonucleotides offers a number of advantages. These advantages include high purity, reproducible yield (independent of RNA sequence and terminal nucleotide), potential for large-scale production and ability to incorporate chemical modifications into the RNA. These triphosphate RNA oligos require dual HPLC or dual PAGE/HPLC purification.
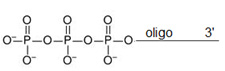
5' Triphosphate Oligo Modification
- 1.0 umole scale: $800/modification
- 5.0 umole scale: $1200/modification
- 10 umole scale: Inquire
- 15 umole scale: Inquire
Above prices do not include synthesis and purification. Prices are valid for oligonucleotides 15-30 bases. For longer oligos, request a quotation.
Large scale also avialable upon request
Reference (s):
- Themed Issue on Biophosphates. New J. Chem. 2010, 5, 769.
- De Clercq, E. Rev. Med. Virol. 2009, 19, 287.
- Miura, S.; Izuta, S. Curr. Drug Targets 2004, 5, 191.
- Ekland, E. H.; Szostak, J. W.; Bartel, D. P. Science 1995, 269, 364.
- Joyce, G. E. Angew. Chem., Int. Ed. 2007, 46, 6420.
- Rohatgi, R.; Bartel, D. P.; Szostak, J. W. J. Am. Chem. Soc. 1996, 118, 3332.
- Perez, J. T.; Varble, A.; Sachidanandam, R.; Zlatev, I.; Manoharan, M.; García-Sastre, A.; tenOever, B. R. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 11525.
- Hornung, V.; Ellegast, J.; Kim, S.; Brzozka, K.; Jung, A.; Kato, H.; Poeck, H.; Akira, S.; Conzelmann, K. K.; Schlee, M.; Endres, S.; Hartmann, G. Science 2006, 314, 994.
- Jemielity, J.; Kowalska, J.; Rydzik, A. M.; Darzynkiewicz, E. New J. Chem. 2010, 34, 829.
5' Adenylated RNA (5-AppRNA)
Bio-Synthesis provides oligonucleotide adenylation using a chemical approach which allows for cost effective scale up of your adenylated oligonucleotide. These adenylated oligonucleotides act as direct substrates for T4 RNA ligase, with the pyrophosphate linkage providing the energy required for the reaction and eliminating the need for ATP1 . It also can be used for applications, including miRNA library construction, next generation sequencing, 5’ end labeling, and ribosome assembly experiments.
Our price includes the addition of the 5′ phosphate to your oligonucleotide, as well as the adenylation itself. To prevent a 5′ adenylated oligo circularized from self-ligating, we recommend to place a blocking group on the 3′-end, such as a 2',3' -dideoxynucleotide or 3' spacer to block the undesired ligation reactions from occurring. Note that this modification requires an extra purification after the adenylation step. This modification is not compatible with other heat-sensitive modifications.
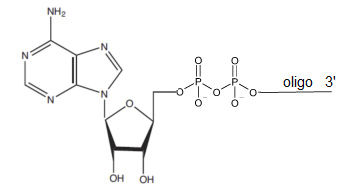
5' Adenylate, rApp, 5' Adenylation
- 1 umole scale: $1250/modification
- 5 umole scale: Inquire
- 10 umole scale: Inquire
Above prices does not include synthesis and purification. Prices are valid for oligonucleotides up to 30 bases. For longer oligos, request a quotation.
Recommendation:
A blocking group (2',3'-Dideoxynucleotide or 3' (C3) spacer ) is recommended to be modified on the 3' end to prevent 5' adenylated oligo from self-ligating.
Large scale also avialable upon request
Reference (s):
- England TE, Gumport RI, and Uhlenbeck OC (1977) Dinucleoside pyrophosphate are substrates for T4-induced RNA ligase. Proc Natl Acad Sci USA, 74:4839–4842.
5' Guanosine Triphosphate oligonucleotide Cap [Gppp]
5' Guanosine triphosphate cap (Gppp), a precursor and unmethylated version of N7-MeGppp cap is often found on the 5' end of eukaryotic cellular and viral messenger RNA and some heterogeneous nuclear RNAs. The cap consists of a methylated GTP linked to the rest of the mRNA by a 5' to 5' triphosphate "bridge". These positively charged structures protect specified RNA at their terminal end against attack by phosphatases and other nucleases. These capping structures also promote mRNA function at the translation level. Analogs of the RNA caps (5' Trimethoxystilbene and pyrene cap), which lack the positive charge such as inhibit the initiation of protein synthesis. 5' terminal m7GpppGm or GpppG are more stable than mRNA containing unblocked ppG 5'-ends.
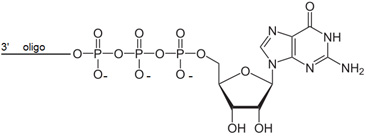
Bio-Synthesis offers chemically synthesized oligonucleotides with 5′ guanosine triphosphate cap. Guanosine Triphosphate oligonucleotides requires PAGE purification and are not compatible with free amine modifications. List pricing is valid for oligonucleotides 5-35 bases.
5' Adenylate, rApp, 5' Adenylation
- 1 umole scale: $1000/modification
- 5 umole scale: $1550/modification
- 10 umole scale: Inquire
- 15 umole scale: Inquire
Above prices do not include synthesis and purification. Prices are valid for oligonucleotides 15-30 bases. For longer oligos, request a quotation.
Reference (s):
- Shatkin, A.J. (1978). Cell. 9, 645-653.
- Fillipowicz, W. (1978). FEBS Lett. . 96, 1-11.
- Banerjee, A.K. (1980). Microbiol. Rev.. 1980, 175-205.
- Miura, K. (1981). Adv. Biophys. . 14, 205-238.
- Shatkin, A.J. et al. (1977). Nucl. Acids Res.. 4, 3065-3081.
- Konarska, M.M. et al. (1984). Cell. 38, 731-736.
- Contreas, R. et al. (1982). Nucl. Acids Res. 10, 6353-6363.
- Paterson, B.M. and Rosenberg, M. (1979). Nature. 279, 696-701.
5' N7-Methylguanosine-triphosphate, 5' N7-MeGppp, 7mGppp, mRNA Cap
The 5´ N7 Methylguanosine triphosphate RNA capping, 5' N7-MeGppp, 7mGppp, mRNA Cap present on most eukaryotic mRNAs promotes in vitro and in vivo translation, at the initiation level. For most RNAs, the methylated RNA cap structure increases stability, decreases susceptibility to exonuclease degradation, and promotes the formation of mRNA initiation complexes. RNA oligonucleotides modified with 5′-N7-MeGppp can mimic cellular capped mRNA and act as an original substrates that can be used for various biochemical and structural studies.
Bio-Synthesis offers N7-MeGppp oligos prepared via chemical synthesis. m7G mRNA cap oligo modification utilized several purification and Q.C methods, including PAGE, reverse phase HPLC, anion-exchange HPLC and mass spectrometry.
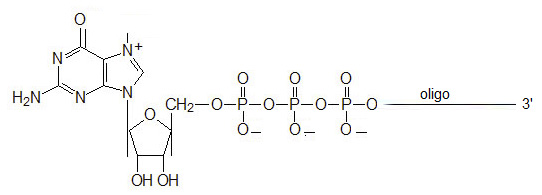
Bio-Synthesis offers chemically synthesized oligonucleotides with 5′ guanosine triphosphate cap. Guanosine Triphosphate oligonucleotides requires PAGE purification and are not compatible with free amine modifications. List pricing is valid for oligonucleotides 5-35 bases.
5' N7-Methylguanosine-triphosphate Cap
- 1 umole scale: $1300
- 10 umole scale: Inquire
Above prices do not include synthesis and purification. Prices are valid for oligonucleotides 15-30 bases. For longer oligos, request a quotation.
Reference (s):
- Shatkin, A.J. (1978). Cell. 9, 645-653.
- Fillipowicz, W. (1978). FEBS Lett. . 96, 1-11.
- Banerjee, A.K. (1980). Microbiol. Rev.. 1980, 175-205.
- Miura, K. (1981). Adv. Biophys. . 14, 205-238.
- Shatkin, A.J. et al. (1977). Nucl. Acids Res.. 4, 3065-3081.
- Konarska, M.M. et al. (1984). Cell. 38, 731-736.
- Contreas, R. et al. (1982). Nucl. Acids Res. 10, 6353-6363.
- Paterson, B.M. and Rosenberg, M. (1979). Nature. 279, 696-701.