Raman Spectroscopy of cytosines modified with a vibrational tag enables the study of protonated cytosine base pairs in oligonucleotides.
A Raman tag is a vibrational tag added to a molecule that allows tracking or probing of protonation states in target molecules. During Raman scattering, an inelastic light scattering process, frequencies of the scattered photons shift from those of the incident photon frequencies. The shift in frequencies occurs according to the vibrational modes of the molecule or atomic group.
DNA i-motifs contain cytosine-hemiprotonated cytosine base pairs [C-H-C]+. These base pairing types can mediate complicated biological functions, including the transfer of genetic information, gene manipulation, and regulation. In i-motifs, an intercalating [C-H-C]+ base pair can form a quadruplex structure even at neutral pH.
Knowing detailed structural information of nucleobases in intercellular nucleic acids and their interaction with the surrounding environment is vital for designing functional molecules useful for oligonucleotide therapeutics. Unfortunately, the protonation state of cytosines in DNA is complicated and remains elusive. Since conventional spectroscopic approaches have some drawbacks, molecular vibration monitored using Raman spectroscopy allows obtaining a structural fingerprint of target molecules.
Itaya expected that the protonation of nucleic acids in oligonucleotides affects their vibrational modes. The research group, therefore, prepared a Raman tag as a vibrational tag by adding a phenyl-acetylene group to a cytosine. The protonation of this tag on the N3 position of cytosine shortens the acetylene bond.
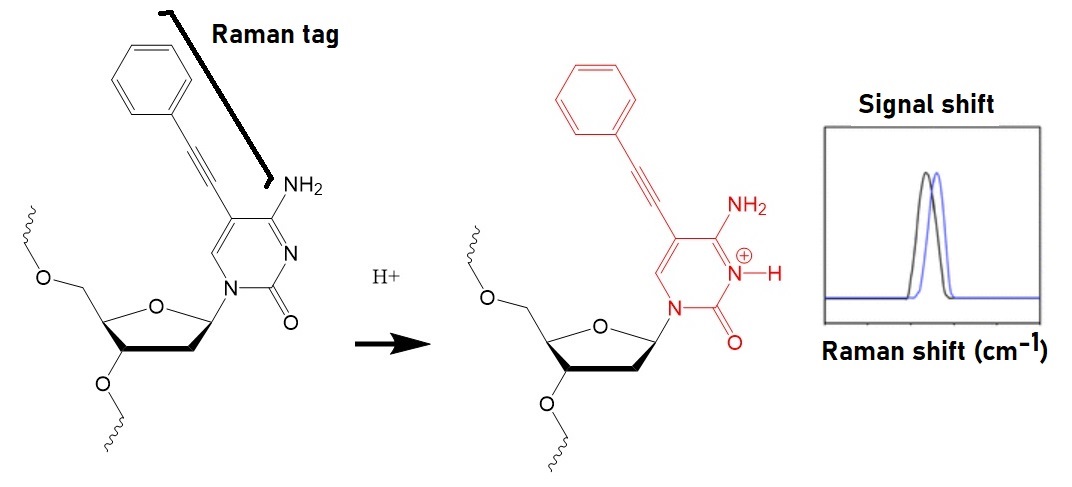
Figure 1: Identification of protonation in a Raman-tagged cytosine oligonucleotide (Itaya et al. 2021). Protonation of the tagged cytosine results in a frequency shift of the Raman-tag.
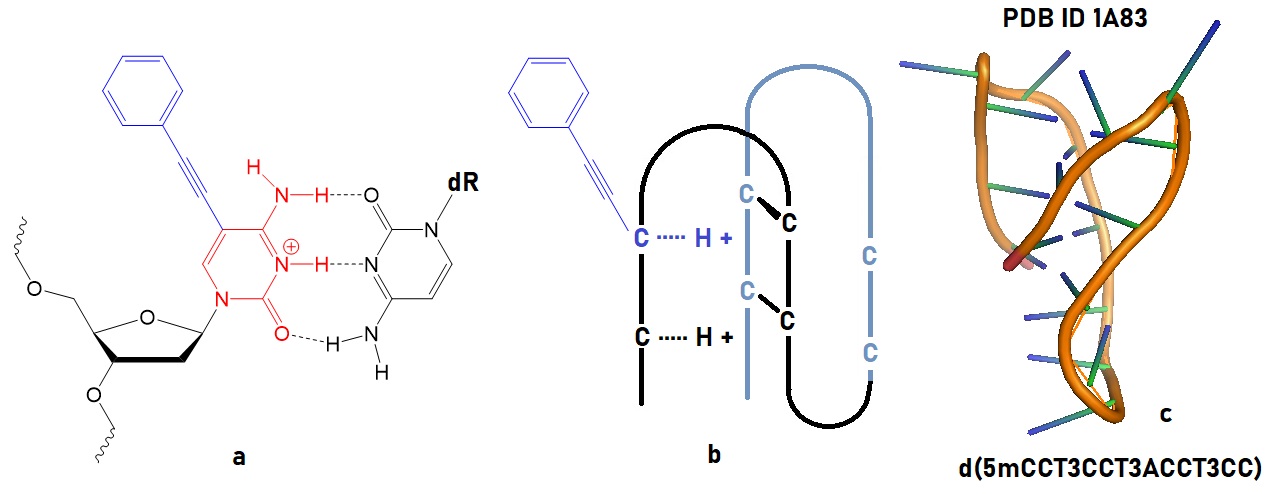
Figure 2: Illustration of an oligonucleotide with a cytosine containing a Raman tag. a) The chemical structure of a C-C+ pair with a tagged C is shown. b) Conceptual drawing of an i-motif oligonucleotide containing the Raman tag. c) Intramolecular i-motif structure solved by NMR (Han et al. 1998; 1a83). Protonation of C-C base pairs leads to formation of an i-motif in oligonucleotides.
When protonated, cytosine can adopt specific structures allowing the formation of higher order structures.
Examples are the formation of:
[1] C+–G–C triplets with one protonated cytosine that interacts with a G–C base pair to form a triplex, and
[2] cytosine·hemiprotonated cytosine base pairs ([C–H–C]+) found in DNA i-motifs.
However, determining the correct protonation status of C in complicated environments is quite tricky. Raman or vibrational tags enable specific detection of chemical species by circumventing the endogenous cellular background. Raman tags can be designed and synthesized by adding chemical bonds to chemical compounds that vibrate in the cell-silent Raman window between 1800 to 2600 cm−1, in which no other endogenous molecules vibrate.
Reference
Han X, Leroy JL, Guéron M. An intramolecular i-motif: the solution structure and base-pair opening kinetics of d(5mCCT3CCT3ACCT3CC). J Mol Biol. 1998 May 22;278(5):949-65. doi: 10.1006/jmbi.1998.1740. PMID: 9600855 [Pubmed].
Ryota Itaya, Wakana Idei, Takashi Nakamura, Tatsuya Nishihara, Ryohsuke Kurihara, Akimitsu Okamoto, and Kazuhito Tanabe; Changes of C≡C Triple Bond Vibration that Disclosed Non-Canonical Cytosine Protonation in i-Motif-Forming Oligodeoxynucleotides. ACS Omega 2021, 6, 47, 31595–31604 [ACS]
Jones RR, Hooper DC, Zhang L, Wolverson D, Valev VK. Raman Techniques: Fundamentals and Frontiers. Nanoscale Res Lett. 2019 Jul 12;14(1):231. doi: 10.1186/s11671-019-3039-2. PMID: 31300945; PMCID: PMC6626094 [PMC].
---...---
Bio-Synthesis provides a full spectrum of oligonucleotide and peptide synthesis including bio-conjugation services as well as high quality custom oligonucleotide modification services, back-bone modifications, conjugation to fatty acids and lipids, cholesterol, tocopherol, peptides as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---