The nucleoside analogue 3-cyanovinyl carbazole, CNVK, enables ultrafast reversible photo-cross-linking of oligonucleotides.
Recently a new non-enzymatic and non-invasive technologies for nucleic acid editing has been reported that takes advantage of the photo-cross-linker 3-cyanovinyl carbazole (CNVK). 3-Cyanovinyl carbazole (CNVK) when incorporated into oligonucleotides photo-cross-links a target pyrimidine and the CNVK to afford deamination of cytosine converting it to uracil.
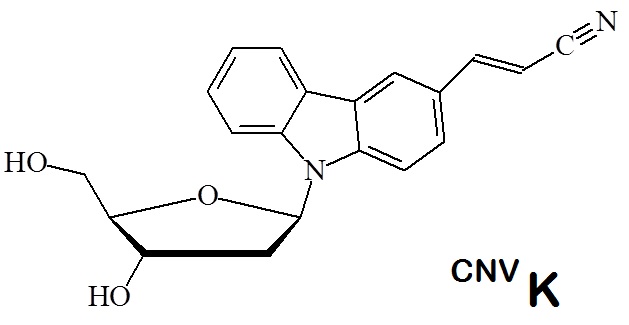
Figure 1: 3-Cyanovinyl carbazole (CNVK) nucleoside analog. This nucleoside is now available as a cyanoethyl (CE) phosphoramidite allowing the incorporation of the analog into oligonucleotides during automated solid phase oligonucleotide synthesis.
Cross-linking and photo-cross-linking reactions are used widely in biochemistry, biology, chemistry, and molecular biology for the study of molecular interactions. Many new cross-linking reagents have been developed and synthesized in the last two decades. Advances made in analytical instrumentation now allow the analysis of cross-linked products at ever lower levels of detection. For example, various photo-cross-linking approaches can be used to study the interactome of cells.
The interactome refers to the network of molecular interactions in living cells, such as protein-protein, protein-nucleic acid, or protein-carbohydrate interactions.
Photo-cross-linking is used fo the study of protein-protein or protein-oligonucleotide interactions in living cells. In this approach, first photo-cross-linkers are incorporated in the desired proteins or oligonucleotides within cells, next, cells are exposed to UV radiation to induce the cross-linking between molecules in contact to the cross-linking functional groups. Following cross-linking, the target molecule of interest and its cross-linked complexes are isolated using immune-purification and analyzed using mass spectrometry. Often enzymatic digestions are used to allow for easier detection of the cross-linked products and to define molecular interphases.
The new photo-cross-linker 3-cyanovinylcarbazole (CNVK) nucleoside allows the study of oligonucleotide interactions.
Yoshimura and Fujimoto in 2008 descibed a ultrafast reversible photo-cross-linking reaction for the manipulation of DNA in situ using CNVK which was applied to the selection of RNA in 2009 by the same research group.
When CNVK is incorporated into oligonucleotides, a very rapid cross-link is formed to a pyrimidine base on the complementary strand when irridated at 366 nm.
According to Yoshimura and Fujimoto, photo-cross-linking to thymine takes only 1 second but takes 25 second to cytosine.
Complete reversal of the cross-link is achieved by irridation at 312 nm for 3 minutes.
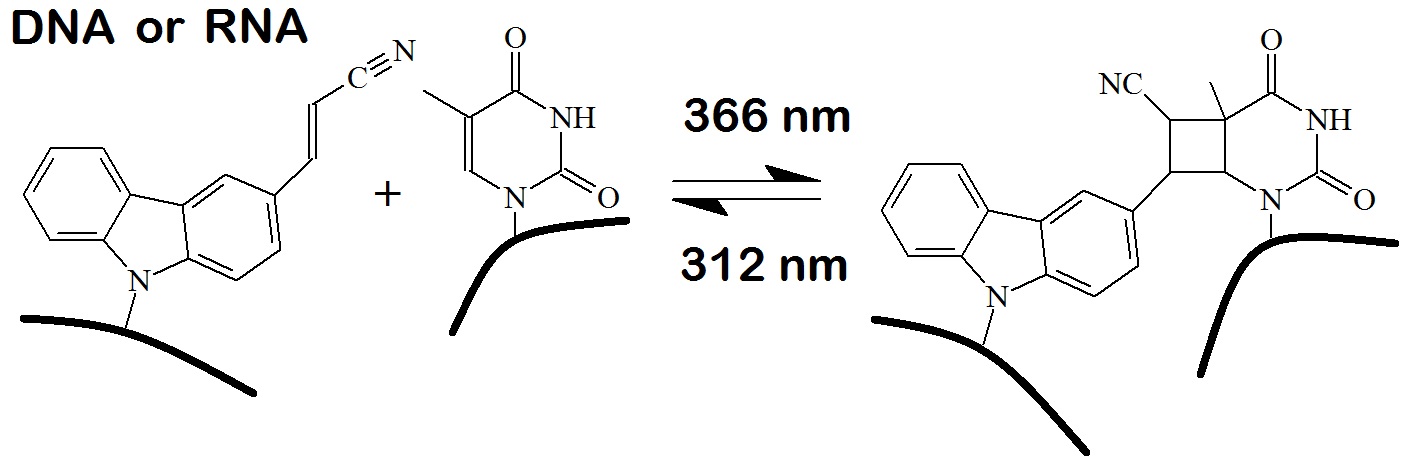
Figure 2: Photo-cross-linking reaction of the 3-Cyanovinyl carbazole (CNVK) oligonucleotide with a thymidine on a complementary oligonucleotide strand. The photoadduct CNVK-T is shown as the reaction product as described by Yohimura and Fujimoto in 2008 and 2009.
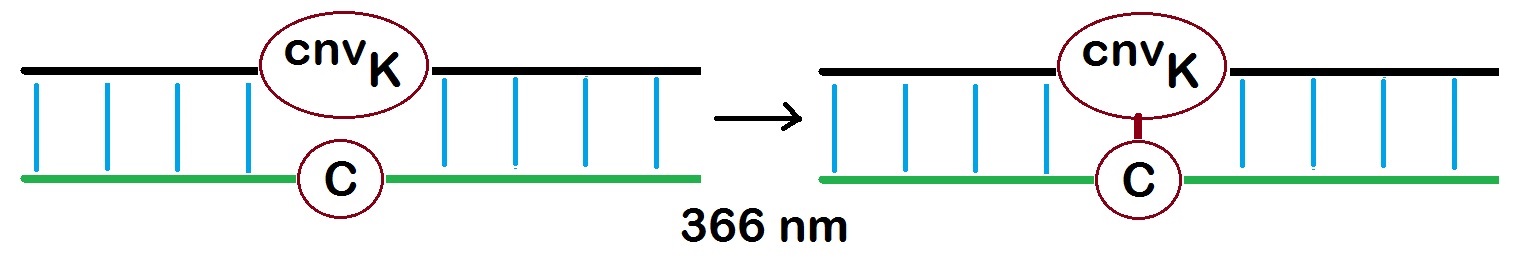
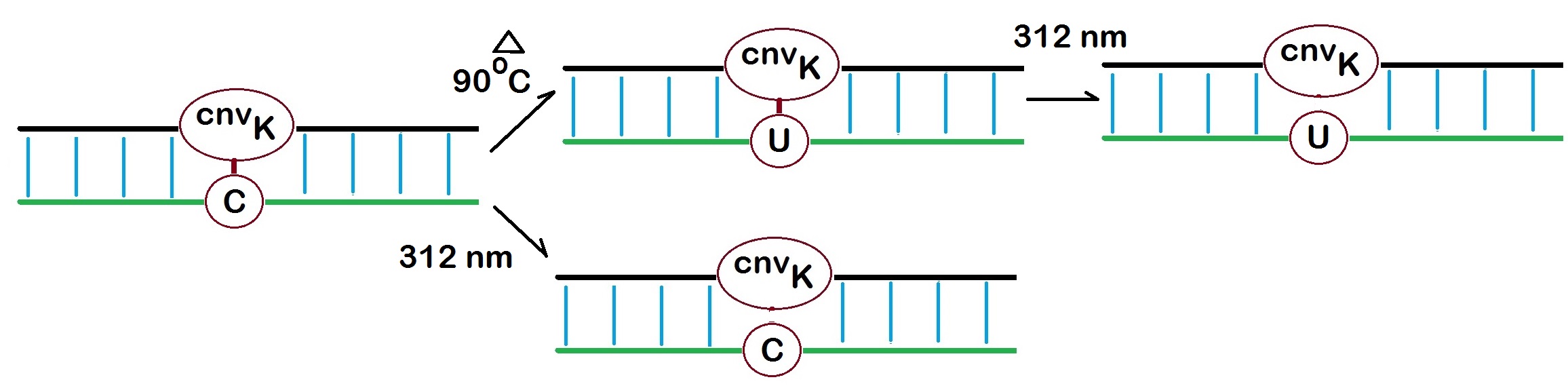
Figure 2: Photo-cross-linking reaction of the 3-Cyanovinyl carbazole (CNVK) oligonucleotide with a cytosine at 366 nm. Heading the cross-link to 90 oC for 3.5 hours causes deamination of the cytosine to form a uracil in the complementary strand (Yoshimura et al. 2009). Reversal of the cross-link at 312 nm results in a complementary strand with a uracil in place of the cytosine.
In 2010 Fujimoto et al. reported the cross-linking reaction of CNVK incorporated into oligonucleotides to a dC residue in duplex DNA. The heating of the cross-link to 90 °C for 3.5 hours resulted in a deamination reaction of the cytosine base now forming a uracil moiety in the complementary strand. Reversal of the cross-link at 312 nm resulted in a DNA strand in which dC is replaced with dU. The transformation is specific for a dC residue opposite a CNVK residue. However, any further adjacent dC residues are unaffected by the reaction. Also, oligonucleotides containing CNVK residues can be cross-linked to adjacent RNA strands.
In 2013, this approach was applied to SNP-based genotyping and in 2015 a threoninol linker was introduced in the oligonucleotide strand.
Sethi et al. in 2018 reported that the ultrafast reversible DNA inter-strand photo-cross-linking reaction of oligodeoxynucleotides (ODNs) containing CNVK can take place at physiological conditions. in order to find the best combination of photo-active nucleobase and complementary base of the target, a variety of combinatios were studied. The study concluded that the best combination of counter-base and photo-cross-linker will result in the highest conversion of C→U and in the least time at physiological conditions.
Reference
2008. Y. Yoshimura, and K. Fujimoto; Ultrafast reversible photo-cross-linking reaction: toward in situ DNA manipulation. Org Lett. 2008 Aug 7;10(15):3227-30. doi: 10.1021/ol801112j. Epub 2008 Jun 27.
2009. Y. Yoshimura, T. Ohtake, H. Okada, and K. Fujimoto; A new approach for reversible RNA photocrosslinking reaction: application to sequence-specific RNA selection. ChemBioChem, 2009, 10, 1473-6.
2010. Fujimoto K1, Konishi-Hiratsuka K, Sakamoto T, Yoshimura Y.; Site-specific cytosine to uracil transition by using reversible DNA photo-crosslinking.Chembiochem. 2010 Aug 16;11(12):1661-4. doi: 10.1002/cbic.201000274.
2012. Wong and Jameson; Chemistry of protein and Nucleic Acid Cross-Linking and Conjugation. 2nd Edition. CRC Press. 2012.
2013. Kenzo Fujimoto, Asuka Yamada, Yoshinaga Yoshimura, Tadashi Tsukaguchi, and Takashi Sakamoto; Details of the Ultrafast DNA Photo-Cross-Linking Reaction of 3-Cyanovinylcarbazole Nucleoside: Cis–Trans Isomeric Effect and the Application for SNP-Based Genotyping. J. Am. Chem. Soc., 2013, 135 (43), pp 16161–16167. DOI: 10.1021/ja406965f. Publication Date (Web): October 2, 2013. Copyright © 2013 American Chemical Society kenzo@jaist.ac.jp :J. Am. Chem. Soc. 135, 43, 16161-16167
2013. Pham, N. D., Parker, R. B., & Kohler, J. J. (2013). Photocrosslinking approaches to interactome mapping. Current Opinion in Chemical Biology, 17(1), 90–101. http://doi.org/10.1016/j.cbpa.2012.10.034.
2015. Sakamoto T, Tanaka Y, Fujimoto K.; DNA photo-cross-linking using 3-cyanovinylcarbazole modified oligonucleotide with threoninol linker. Org Lett. 2015 Feb 20;17(4):936-9. doi: 10.1021/acs.orglett.5b00035. Epub 2015 Feb 5.
2018. Sethi, S.; Nakamura, S.; Fujimoto, K.; Study of Photochemical Cytosine to Uracil Transition via Ultrafast Photo-Cross-Linking Using Vinylcarbazole Derivatives in Duplex DNA. Molecules 2018, 23, 828.
---...---