Characterization of Cyclic, Stapled, and Branched Peptides via Mass Spectrometry
Cyclic, stapled, and branched peptides are a class of biomolecules with promising therapeutic properties. Stapled and cyclic peptides are non-linear peptides that have shown biostability and resistance to proteolytic digestion in physiological environments. Peptides protected from degradation have been shown to exhibit high potency and low toxicity. As a result cyclic, stapled peptides are now considered as promising candidates for therapeutics. Nature also provides us with nonlinear peptides, some of which inhibit enzymes. The Bowman-Birk class of protein inhibitors, Sunflower trypsin inhibitor-1 (SFTI-1) and Momordica cochinchinesis trypsin inhibitor-II (MCoTI-II) are examples of enzyme inhibiting peptides.
However, the structural characterization of cyclic and stapled peptides during their analysis turned out to be not trivial. For example, elucidating the sequence of a cyclic peptide is much more difficult than for a linear peptide. Recently researchers at the University of Texas at Austin, Texas, the Southwestern University in Georgetown, Texas, and Bristol-Myers Squibb in Princeton reported a mass spectrometry-based method for the characterization of cyclic or cyclic-stapled peptides. This new method employs an arginine-selective derivatization strategy in combination with the analysis of the modified peptide via tandem electro-spray mass spectrometry (ESI-MS/MS). Arginine residues present in the cyclic or stapled peptides were converted to ornithine residues with the help of a deguanidination reaction. This reaction converts the arginine residue into a highly selectively cleavable site in the peptides. The use of collision-induced-dissociation (CID) or a similar type of activation induces the ornithine residue to cyclize and to break the adjacent amide bond. This phenomenon is now known as the “ornithine effect”.
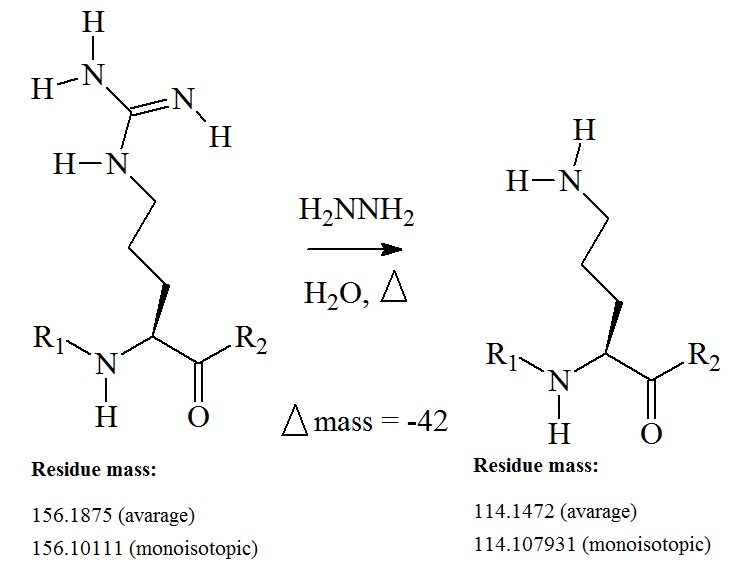
Conversion of an arginine residue to ornithine via deguanidination
The amino acid ornithine is not coded by DNA. Ornithine is produced in nature via de-guanidination of arginine. Ornithine plays an important role in the urea cycle. The chemical conversion of arginine to ornithine results in a δ-amino-containing side chain. The proton affinity of the ornithine side chain is expected to be very similar to that of lysine but much less of that of arginine. This change is expected to alter the fragmentation pattern in a polypeptide in which the ornithine residue is present. The side chain ornithine introduces a neighboring group effect which leads to a highly selective cleavage C-terminal to the ornithine residue. The conversion of arginine to ornithine in a peptide or protein introduces a highly selective decomposition route during peptide fragmentation in a mass spectrometer. This is now known as the "ornithine effect."
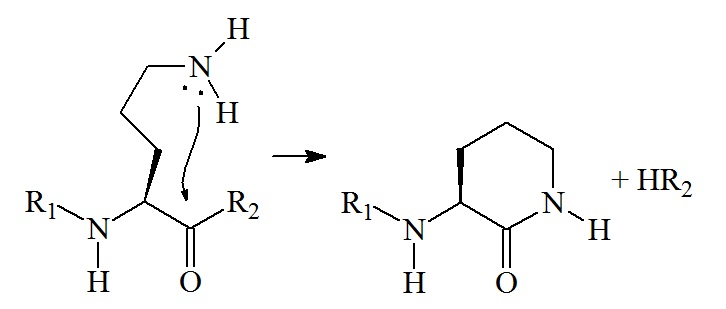
Conversion of ornithine residue to a lactam ring
The nucleophilic attack of the carbonyl carbon by the amine results in the formation of a six-membered lactam and the cleavage at the C-terminal carbonyl carbon bond od ornithine. If the ornithine residue is an N-terminal or internal residue, HR is an amine (HNH-R). If the ornithine residue is a C-terminal residue, HR is a water loss (H2O). The formation of a six-membered lactam gives rise to an abundant b ion.
Synthesis of cyclic, stabled peptides
Different biochemical strategies allow for the biological synthesis of circular or backbone-cyclized proteins and peptides. These methods employ either in vitro or in vivo using standard recombinant DNA expression techniques for the production of circular polypeptides. However, circular or backbone-cyclized peptides that contain a bridge introduced by stapling are synthesized using standard solid phase peptide synthesis methods. Synthetic cyclic, stapled peptides are produced using Fmoc solid phase peptide synthesis. Usually, a ring-closing metathesis reaction is used. Typically a catalyst such as the Grubbs’ First Generation Catalyst is employed for the cyclization reaction. Finally, for the production of a clean product, the resulting peptides are purified by preparative reversed-phase HPLC and the purity is confirmed by analytical HPLC and mass spectrometry.
The ornithine effect in mass spectrometry
The ornithine effect has been reported as a site-specific cleavage occurring C-terminal to ornithine residues in peptides in the gas-phase of a mass spectrometer.
During the activation of an ornithine-containing peptide in a mass spectrometer via collision activation the amine of the ornithine residue cyclyzes via nucleophilic attack at the adjacent carbonyl group. The result is a characteristic and preferential cleavage C-terminal to the carbonyl group. This is called the "ornithine effect."
The ornithine effect can be exploited for the production of unique fragment ions that allow for the differentiation of isomers in unmodified, modified, cyclic and stapled peptides. However, a list of every possible combination of bond cleavage including cross-ring cleavages with the associated masses of fragments will need to be created to allow assignment to the experimental detected fragment ions.
Reference
Christopher M. Crittenden, W. Ryan Parker, Zachary B. Jenner, Kerry A. Bruns, Lucas D. Akin, William M. McGee, Eugene Ciccimaro, and Jennifer S. Brodbelt; Exploitation of the Ornithine Effect Enhances Characterization of Stapled and Cyclic Peptides. J. Am. Soc. Mass Spectrom. (2016) Published on line: 10 February 2016.