Gapmer Design
A gapmer is a chimeric antisense oligonucleotide that contains a central block of deoxynucleotide monomers sufficiently long to induce RNase H cleavage.
General Design
The central block of a gapmer is flanked by blocks of 2’-O modified ribonucleotides or other artificially modified ribonucleotide monomers such as bridged nucleic acids (BNAs). In a gapmer these modified nucleic acids protect the internal block from nuclease degradation. Natural unmodified DNA, as well as modified DNA analogs such as phosphorothiote DNA analogs can be used to stabilize RNA molecules useful as gapmers for therapeutic approaches.
Design Scheme
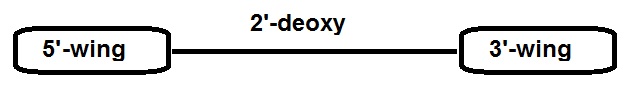
|
5’-wing
|
gap
|
3’-wing
|
|
|
|
|
|
2 to 3 BNAs
|
8 to 12 natural nucleic acids, or
phosphorothioate (PS) nucleic acids
|
2 to 3 BNAs
|
|
|
5’-NNnnnnnnnnnnNN-3’
14mer
|
|
|
|
d(CUTAGCACTGGCCU)-3’
2’,4’-BNANC[NMe]
Target: PTEN
|
|
Antisense Technology
Antisense technology is a universal approach for the inhibition of gene expression in a sequence-specific manner. Since the discovery that oligodeoxynucleotides can act as antisense agents that inhibit viral replication in cell culture antisense technology has been developed for target validation and therapeutic purposes. Theoretically, antisense approaches can be used to cure any disease caused by the expression of deleterious genes, including diseases caused by viral infections, cancer growth, and inflammatory diseases. Antisense technology allows inhibition of gene expression offering itself as a tool for the study of gene function (functional genomics) as well as for therapeutic approaches (antisense gene therapy). However, in practice antisense technology has proved to be challenging.
In general, if the RNA sequence is known, the antisense sequence is easy to work out. However, to achieve success, length, chemical modifications and target site need to be carefully selected.
Optimized antisense oligonuclotides (ASOs) such as gapmers support RNase H and directly cause the decay of RNA targets.
Identify molecular targets – it starts with a gene
A gene of interest maybe selected due to its known biological function in diseases related pathways or because little is known and functional genomics studies are planned.
Gapmers can be used to target primary gene transcripts, mRNA product(s), spliced and unspliced coding and noncoding RNAs. Alternative starts of transcription and alternative polyadenylation leading to alternative primary transcripts will need to be taken in account when designing ASOs that target variants. Usually, the design of ASOs including gapmers starts with a gene. The literature can provide information for many genes. Alternatively, public sequence databases such as Genbank or Pubmed are often the best sources. Sequence data from next generation sequencing (NGS) projects are also a good source for molecular targets. For the design of ASOs or gapmers that target only one member of a gene family, specific sequences will need to be identified. Homology searches versus databases of RNAs in the same species can be used for the identification of cross-reacting RNAs. Any gene that even has a weak homology to the target gene is a potential cross-reactor. Alignments of complementary DNAs (cDNAs) and expressed sequence tags (ESTs) can be done using search algorithms such as the Basic Local Alignment Tool (Blast) or similar tools (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Targets for ASOs may be found in untranslated regions (UTRs), coding sequence (CDS), introns, splice sites (intron:exon and exon:intron junctions), and exon:exon junctions.
Binding of antisense oligonuclotides (ASOs)
For optimal or total binding of an ASO to an RNA target the self-structure in the ASO must be removed and secondary structure in the RNA target must be opened up. The ASO will then bind to the denatured target site according to its favorable free energy.
What to avoid?
- Avoid sequence motives associated with non-antisense activities:
- The treatment of rodents or primates with ASOs containing motifs with unmodified CpG can result in immunostimulation.
- Strings of guanosine can result in nonantisense activity.
- For the identification of potential mismatch sites the alignment tool FASTA can be used.
- Avoid sites of polymorphisms.
- Avoid cross-reactive sequences.
- Avoid potential aptameric motifs.
Unfortunately, to identify all potential mismatch sites, one would need to test each candidate ASO versus the entire transcriptome.
Success Rate of ASO screens
Freier and Watt reported in 2008 (in “Antisense Drug Technology”) that a screen using 10 to 20 ASOs in vivo can identify compounds with good activity. Data from a screen using a set of 21 ASOs targeting a single mRNA in mouse kidney identified four (4) active ASOs. A screen using 57 ASOs targeting a single gene resulted in various “hit rates.” Approximately one third of the compounds tested reduced targeted mRNA below 40% control, 12% reduced it below 25% control, but only 7% reduced it below 20% control. In this example, four candidates with activity below 20% control where identified.
Therefore, for a decent target validation screen, a minimum set of three (3) to six (6) designs will be needed.
If possible, positive and negative controls should be used.
In general, using more ASOs for a screen will increase the number of identified active ASOs.
Design of BNANC gapmer oligonucleotides
A general gapmer design consists of a 5’-wing followed by a gap of 8 to 12 deoxynucleic acid monomers that may be natural nucleic acids or contain a sulphur ion in the phosphor group (PS linkage) followed by a 3’-wing. This is a RNA-DNA-RNA-like configuration (e.g. 2-10-2).
PS linkages are known to improve stability of the gapmer while maintaining its ability to elicit RNase H activity. Also, they contribute to protein binding properties that prevent rapid excretion and facilitate uptake to tissues.
BNA Gapmer Design Example
2’,4’-BNANC[NMe] PTEN
5-NNnnnnnnnnnnNN-3’
d(CUTAGCACTGGCCU)3’
2’,4’-BNANC[NMe] PTEN
.jpg)
This design supports the enzymatic activity of RNase H cleavage of the targeted mRNA without the metabolic degradation due to exonuclease-mediated trimming of the termini.
Prakash et al in 2010 (Prakash, T.P. et al. (2010) J. Med. Chem. 53, 1636–1650.) showed that BNA modified gapmers worked well in mice (liver): 2',4'-BNANC[NMe] worked best, then N-MeO-amino BNA, and then N-Me-aminooxy BNA. A fourth modification, 2',4'-BNANC[NH], worked well in cell culture but not by systemic delivery in mice. N-MeO-amino BNA, 2’4’-BNANC, and 2’4’-BNANC-[NMe] containing ASOs (14-mer 2-10-2 gapmer) showed high affinity to target RNA, significantly higher than the corresponding MOE ASO. However, it is not clear at this point in time that the chemistry that worked well in liver will work in muscle. The artificial nucleotide 2',4'-BNANC[NMe] is the derivative of choice for the design of gapmers since it appears to have worked the best.
Modified nucleic acids used in ASOs
Several artificial nucleic acids have been investigated for their use in antisense technology. Chemical structures investigated in a study published by Prakash et. al. in 2010 are shown below.
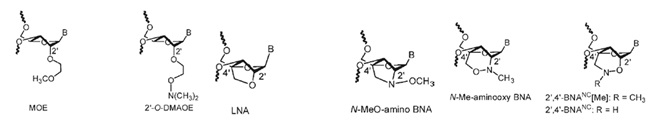
Figure 1: Artificialy modified nucleic acids used in antisense technology.
Legend: MOE, 2,-O-(2-methoxyethyl); BNA, 2’,4’-bridged nucleic acid; LNA, locked nucleic acid; N-Me-aminooxy BNA, 2’-N-(methyl)-4’-C-aminooxymethylene 2’,4’-bridged nucleic acid; N-Me-aminooxy 2’,4’-bridged nucleic acid, 2’-N-(methyl)-4’-C-aminooxymethylene 2’,4’-bridged nucleic acid; 2’,4’-BNANC [NMe], 2’-O,4’-C-(N-methyl) aminomethylene 2’,4’-bridged nucleic acid; 2’,4’-BNANC, 2’-O,4’-C-aminomethylene 2’,4’-bridged nucleic acid.
Open questions in regard to mechanism
However, a few fundamental questions that influence the use of gapmers still need to be answered.
- Exact mechanisms for distribution of gapmers or any ASO out of plasma and the accumulation in cells are still unknown.
- Molecular pathways ASOs use for selective binding to receptors inside cells need to be studied in more detail.
- Many enzymes responsible for the degradation of ASOs in plasma and tissue are unknown.
- Factors that limit gapmers and ASOs from crossing mucosal membranes in the intestine and other mucosal membranes need to be investigated as well.
- Duration of action for gapmers or ASOs used will need to be established.
Delivery in to cells via transfection
Selection of delivery method
- Use a transfection reagent.
- Usually cell are transfected using Lipofectin (Invitrogen, Carlsbad, CA). Other delivery methods may include the use of lipid or amine based transfection reagents, as well as electroporation.
- Unassisted uptake is also possible. Higher concentrations of gapmers will be needed (up to 5 µM) and the uptake will occur more slowly.
Controls
- Positive gapmer controls provide validation of antisense approaches.
- Negative controls similar to designed gapmers are recommended for their use.
- Untreated controls allow evaluation of transfection not related to gapmer antisense activity.
Gapmer uptake may be monitored using fluorescently labeled gapmers.
How to find the best BNA gapmer
- Use 3 to 6 different BNA gapmer designs for the identification of potent knockdown gapmers.
- Use the lowest effective concentration. To find out, perform a dose response study. Recommended concentration ranges for BNA gapmer transfection is 0.1 to 100 nM, or 100 nM to 5 M for unassisted uptake.
- Monitor timecourse of knockdown. This will depend on the delivery method, the stability and turnover of the transcript. Most gapmers target primarily newly synthesized RNA transcripts in the nucleus. Sometimes it may take longer to observe an effect if highly stable cytoplasmic transcripts or protein complexes are used.
- The use of a second BNA gapmer is recommended for the validation of the phenotype. To verify a knockdown effect more conclusively, the use of at least two gapmers targeting different sequence positions within the target RNA including a negative BNA gapmer are recommended.
In vitro test prior to in vivo use of BNA gapmers
- Screening multiple BNA gapmers in vitro increases the likelihood for a successful knockdown experimentin vivo. This is important if the goal is to achieve maximal silencing of the target RNA in vivo.
- Test unassisted uptake of BNA gapmers in vitro. Using a representative cell line of the target tissue successful uptake can be simulated, thus allowing for a better prediction of results.
- Consider starting with an in vivo pilot experiment to identify optimal dose range. Knockdown efficacy and duration of effect in relevant tissues can be monitored using a limited number of animals and test range doses via subcutaneous administration.
- Signs of toxicity can be monitored by checking liver and kidney function, for example by testing blood for aspartate aminotransferase/alanine aminotransferase (AST/ALT) transaminases and creatinine/urea.
Reference
Stanley T. Crooke (Editor); Antisense Drug Technology- Principles, Strategies, and Applications. 2n Editon. CRC press. 2008.
Thazha P. Prakash, Andrew Siwkowski, Charles R. Allerson, Michael T. Migawa, Sam Lee, Hans J. Gaus, Chris Black, Punit P. Seth, Eric E. Swayze, and Balkrishen Bhat. Antisense Oligonucleotides Containing Conformationally Constrained 2’,4’-(N-Methoxy)aminomethylene and 2’,4’-Aminooxymethylene and 2’-O,4’-C-Aminomethylene Bridged Nucleoside Analogues Show Improved Potency in Animal Models. J. Med. Chem. 2010, 53, 1636–1650. [PubMed]
-.-