Peptide nucleic acid (PNA ) is an artificially synthesized polymer that is capable of binding DNA and RNA in a sequence-specific manner. Since the discovery of its unique binding properties, PNA has been employed in a wide variety of biomedical applications, including genetic research, diagnostics, and experimental therapeutics (1). This article will focus on the diagnostic PNA assays that have gained widespread use in the pathology setting and briefly touch upon other promising applications of this technology.
Unlike DNA and RNA , which have backbones of repeating sugarphosphate units, the PNA molecule is built upon a pseudo-peptide backbone of N-(2-aminoethyglycine) units linked by peptide bonds, to which purine and pyrimidine bases (the specific base-pairing units of nucleic acids) are linked via methylene carbonyl bonds.
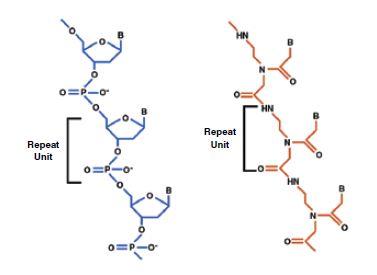
Figure 1. Basic structures of DNA and PNA. In contrast to the negatively charged backbones of naturally occurring nucleic acids (DNA and RNA), the synthetic pseudo-peptide backbone of PNA is uncharged. PNA’s electrostatically neutral backbone is responsible for the higher binding energies (and correspondingly higher melting temperatures) of complementary PNA-DNA and PNA-RNA duplexes relative to similar DNA-DNA, DNA-RNA, or RNA-RNAs. The same bases that are responsible for the sequence-specific base-pairing of nucleic acid duplexes may also be incorporated into PNA. B = pyrimidine (T, C) or purine (A,G) base; H = hydrogen; N = nitrogen; O= oxygen; P= phosphate.
The most common usage for PNA molecules are as probes of complementary nucleic acid sequences. As with other nucleic acid probes, the sequences of bases on PNA probes dictate the specificity of binding to complementary DNA and RNA sequences, but the uncharged PNA backbone confers a key advantage to PNA probes. By eliminating the repulsive electrostatic force between traditional nucleic acid probes and their complementary target strands, the neutral PNA backbone confers increased probe affinity and thermal stability to the probe-target duplex.
Specificity of probe binding is a critical aspect of probe assay design, and the physico-chemical properties of PNA probes offer significant advantages for controlling assay specificity. In assays that use traditional DNA or RNA probes, the selectivity conferred by hydrogen bonding between the complementary base pairs on the probe and target strands is offset by the repulsive ionic forces between the strands’ negatively charged backbones. Optimization of assay specificity requires a delicate balance between parameters such as hybridization temperature, probe concentration, length, and G-C content, and the concentrations of organic solvents and ions, making the design of a robust assay challenging even for experienced diagnosticians. The higher binding energies of PNA probe-target duplexes contributed by the uncharged PNA backbone offer several practical advantages for diagnostic probe assay development. The higher melting temperatures of PNA -DNA duplexes allow PNA probes to “invade” and overcome many problematic secondary structures in target sequences, and permit very stringent hybridization and wash conditions to be used to increase binding specificity. The higher binding affinities of PNA probes also permit shorter probe sequences and lower probe concentrations to be used in assays, lowering costs and reducing potential non-specific interactions with assay substrates and biological sample components. Mismatches in PNA-DNA duplexes are more de-stabilizing than in corresponding DNA -DNA duplexes, a characteristic which allows PNA s probes to distinguish single base sequence discrepancies such as point mutations and single nucleotide polymorphisms with higher selectivity than DNA or RNA probes
Another clear benefit of PNA probe chemistry is its exceptional stability. PNA molecules are highly resistant to both nuclease and protease enzymes, and are stable over a wider pH range than DNA or RNA molecules. Probe stability is especially important in diagnostic settings with potentially high amounts of contaminating enzymes, such as assays of minimally processed biological specimens or point-of-use field applications. PNA ’s stability can also be used to advantage in the design of simplified, rapid diagnostic tests which incorporate PNA probes with other assay components, such as sample preparation reagents, in order to consolidate and reduce steps in the assay procedure.
The majority of the commercial PNA probe products available today are designed for fluorescent in situ hybridization (FISH) assays. Dako was an early pioneer in the development of PNA -based tests, and in keeping with its pathology focus is using PNA s to enable novel cancer diagnostics. The first PNA probe diagnostic products on the market were Telomere PNA FISH Kit.

Figure 2. Metaphase spread of human lymphocyte stained with Telomere PNA FISH Kit/Cy3,
These assays, originally conceived and developed by Peter Lansdorp’s group at the Terry Fox laboratory of the British Columbia Cancer Research Center, use PNA probes to quickly and quantitatively visualize the lengths of the telomeric repeat sequences at the ends of each chromosome (2). The kits can be used to assess telomeres in humans and other vertebrate species using interphase nuclei, metaphase spreads, or flow cytometry preparations. Telomere length has been implicated as a critical regulator of a cell’s capacity for division, and the PNA telomere assays have proven to be valuable tools for studying the relationship between telomere length and cancer, senescence, and other events that influence genetic longevity.
More recently, PNA s have been incorporated into a line of cancer cytogenetic FISH probes, where they are used to enhance assay performance. Each of the FISH products, which include both the Split Signal and Sub-Deletion Signal categories of FISH probes, consists of two DNA fragments (labeled with green and red fluorophores, respectively) complementary to adjacent chromosomal regions that are susceptible to re-arrangement in hematological cancers.

Figure 3. The human BCR gene consists of 23 exons spanning a region of 135 kb on chromosome 22 band q11. Y5403 is a probe mix is based on a combination of DNA and PNA technology, and contains two FISH DNA probes and unlabeled PNA blocking probes. The FISH DNA probes are a mixture of a Texas Red-labeled DNA probe (BCR-Upstream) covering 333 kb centromeric to the BCR breakpoint cluster region and a fluorescein-labeled DNA probe (BCR-Downstream) covering 408 kb telomeric to the BCR breakpoint cluster region.
 |  |
| Figure 4. Precursor B-ALL with BCR gene translocation. | Figure 5. Breast carcinoma (FFPE) stained with HER2 FISH. Tumor cells show HER2 gene amplification (HER2/ CEN-17 ratio ≥ 2). |
This line of FISH probes is used for the diagnosis of specific hematologic malignancies — the juxtaposition or separation of the red and green DNA probe signals in the interphase nuclei of cancer cells are indicative of gene re-arrangements that are characteristic of particular leukemia and lymphoma phenotypes. Gene-based testing in this setting can resolve diagnostic ambiguities that often confound traditional immuno-phenotyping methods, but early FISH assays required difficult, lengthy procedures which only a few highly specialized laboratories were able to perform. By incorporating PNA technology into the FISH hybridization probe mixture, it has been possible to simplify and shorten the FISH methodology and broaden its use among pathology labs. These unique probe mixtures contain several unlabeled PNA s that hybridize to the highly abundant repetitive (non-coding) DNA sequences found on human chromosomes. By quenching the non-specific signals from labeled probe binding that ordinarily plague such assays, the PNA s increase the signal-to-noise ratios of the assays and improve the specificity and sensitivity of these tests over what has previously been possible using traditional FISH technology, all in a single hybridization step. A comprehensive product line of two dozen FISH probe mixtures is available, covering the major hematological malignancies.
PNA probe technologies are also employed in the pharmacoDiagnostic® line of FISH kits for determining HER2 and TOP2A gene status in formalin-fixed, paraffin-embedded breast tumor sections. These assays are part of a growing arsenal of highly specific genetic tests which help guide pathologists and oncologists in the diagnosis, prognosis, and selection of treatment for breast cancer. Marketed as the HER2 FISH pharmDx™ and TOP2A FISH pharmDx™ Kits, these assays employ PNA s as both unlabeled and labeled probes in a complex hybridization mixture. The unlabeled PNA s block repetitive sequences, while the fluorescein-labeled PNA s generate green signals that identify centromeric sequences on chromosome 17. The number of green PNA signals in each nucleus determines the copy number of chromosome 17 in the cells. Copy number is likewise obtained for the HER2 or TOP2A gene regions using the red signals from the corresponding HER2 or TOP2A DNA probe, and from the ratio of gene-to-chromosome 17 signals, a numerical value for gene amplification or deletion is derived. The HER2 FISH pharmDx™ Kit is approved by the FDA as an aid in the assessment of breast cancer patients that are being considered for Herceptin® (trastuzumab) therapy. The TOP2A FISH pharmDx™ Kit is approved by the FDA as a marker of poor prognosis in high-risk breast cancer patients.
PNA probes have also been developed to RNA targets specifically for chromogenic ISH detection, with an aim toward improving upon immunohistochemical methods of detection on routinely processed histological sections. PNAs to the Epstein-Barr virus EBER RNAs and the human immunoglobulin light-chain Kappa and Lambda gene mRNA s are the most prominent examples of probes that used for routine bright-field ISH. The EBV EBER probe is a mixture PNA s that detect two nuclear RNA transcripts, EBER1 and EBER2, that are produced by the Epstein-Barr virus in latent infections, including conditions such as Burkitt’s and Hodgkin’s lymphomas, nasopharyngeal carcinomas, and mononucleosis. Since the EBER transcripts are not translated into protein, these unique analytical targets cannot be detected by antibody tests.
The detection of only one of either the kappa or lambda light chain mRNA s in the lymphoid cells of a suspected lymphoma tissue (light chain restriction) is indicative of the monoclonal populations that typify lymphoid malignancies. The use of PNA ISH technology for this application allows the light chain mRNA s to be visualized where they are synthesized in the cytoplasm — an improvement over immunophenotyping by IHC, which can suffer from high levels of background signal contributed by secreted antibodies in serum and interstitial fluids.
Significant advances in the implementation of PNA technology have also been made in other areas of medicine, including:
-
- Microbiology — Commercial assays are now available for identification of Candida, Psuedomonas, Staphylococcus, and Enterococcus sp. in smears made from blood cultures, and several tests for detection of specific genes associated with drug resistance in Staphylococcus aureus isolates are available in microwell format (3, 4).
- Genetic disease testing and research — The scientific literature contains descriptions of the uses and advantages of PNA s in nearly every molecular testing format, as applied to many different genetic diseases (5).
- Gene therapy — PNA has been used to manipulate gene expression in disease models by a variety of techniques including anti-sense, anti-gene, and transcription factor decoy approaches (6).
PNA technology has enabled several complex diagnostic methodologies to be greatly simplified and accelerated, resulting in robust and rapid tests that can be routinely performed in laboratories for many critical diagnostic situations. The compelling practical benefits of PNA probes are continuing to drive the development of new assays that overcome difficult diagnostic challenges and open new paradigms for patient diagnosis and treatment. The rigorous optimization and commercialization of standardized PNA assays will ensure that this promising technology continues to facilitate new diagnostic tests.
References
-
- Egholm M, Buchardt O, Christensen L, Behrens C, Freier SM, Driver DA , et al. PNA Hybridizes to Complementary Oligonucleotides Obeying the Watson-Crick Hydrogen Bonding Rules. Nature 1993; 365:566-8.
- Lansdorp P, Verwoerd N, van de Rijke F, Dragowska V, Little MT, Dirks R, et al. Heterogeneity in telomere length of human chromosomes. Hum Mol Genet 1996; 5: 685-91.
- Forrest GN . PNA FISH: present and future impact on patient management. Expert Rev Mol Diag 2007; 7:231-6.
- Marketed by AdvanDx Inc.
- Pellestor F, Paulasova P, Hamamah S. Peptide nucleic acids (PNA s) as diagnostic devices for genetic and cytogenetic analysis. Curr Pharm Des. 2008; 14:2439-44.
- Karkare S, Bhatnagar D. Promising nucleic acid analogs and mimics: characteristic features and applications of PNA , LNA , and morpholino. Appl Microbiol Biotechnol. 2006 ;71:575-86.