Cell-Penetrating or “Trojan” Peptides
Cell-penetrating peptides or CPPs are short peptides of less than forty (40) amino acids. As the name “cell-penetrating peptides” indicates, these peptides enter cells by passing through hydrophobic cellular membranes. Several possible mechanisms for how cell-penetrating peptides can enter cells have were proposed and reviewed in the literature since the discovery of the first cell-penetrating peptide ‘HIV TAT transactivating factor’ in 1988. Unique features of CPPs are that they can enter cells by translocating through cell membranes. Many earlier CPPs are truncated versions of full-length proteins. The peptides penetratin and TAT are examples. However, over time more complex CPPs have emerged, some of which contain whole synthetic peptide sequences.
Endocytosis is considered to be the primary mechanism facilitating the intra-cellular delivery of CPPs that can also contain covalently or noncovalently conjugated cargo molecules.
Endocytosis is an energy-dependent cellular process engulfing different molecules to allow for the transport of solid or liquid matter into a cell via coated vesicles or vacuoles. Translocation is thought to occur via several pathways divided into four types: clathrin-mediated endocytosis, caveolae -mediated endocytosis, macropinocytosis, and phagocytosis.
Research performed during the last 20 years involving cell-penetrating peptides showed that these peptides are useful for several therapeutic applications. CPP-cargo conjugates are considered to be useful for therapeutic applications such as the treatment of cancer, muscular dystrophy, stroke, be used as anti-prion, anti-viral, and anti-bacterial agents. In addition, during these years it has been observed that a many peptides and proteins are able to penetrate cell membranes and enter cells. Furthermore, it has been shown that even many cargo molecules that are covalently attached to these peptides will be translocated into the cell. Peptides that show the ability to translocate through the cell membrane are usually short peptides of less than 30 amino acids. Their only common feature appears to be that they are amphipathic and have a overall positive net charge. The exact mechanism of cell translocation is not exactly known but appears to be receptor and energy independent, although in some cases their translocation can be partially mediated by endocytosis and various mechanisms have been discussed in the literature. The penetration into cells is usually rapid and of first-order, with half-times from 5 to 20 minutes [Zorko, M., and Langel, U., 2005].
The ability of a 60-amino acid polypeptide corresponding to the sequence of the Drosophila antennapedia gene homeobox to traverse across cell membranes of nerve cells and accumulate in the nuclei was first reported by Joiliot et al, in 1991.
A shorter peptide coined penetratin is a 16 amino acids long peptide of cationic nature containing the sequence: RQIKIWFQNRRMKWKK. This sequence derives from helix 3 of the antennapedia complex [Thoren, P.E., et al., 2003; Fischer, P.M., et al., 2000] and is able to translocate through the plasma membrane to the cytosol and nucleus of living cells, both at 37 °C and 4 °C respectively.
More recently, methods have been developed for the delivery of exogenous proteins into living cells with the help of membrane-permeating carrier peptides derived from HIV-1 Tat (residues 48 to 60) and antennapedia (residues 43 to 58), penetratin [Derosssi, D., et al., 1998; Dunican D.J., et al., 2001]. The basic nature of these peptides and the locations of aromatic groups within their sequence allows these peptides to penetrate the cell membrane.
Investigating a range of basic peptides, Futaki reported that a peptide containing eight arginine residues can efficiently translocate across the cell membrane [Futaki, S. 2002]. Recently, a chimeric peptide derived from galparan and transportan has been used as an effective peptide vector for bio-delivery of PNA molecules [Pooga, Marcus., et.al., 1998]. Translocation of the penetratin peptide occurs even when it is coupled to hydrophilic molecules (e.g. phosphopeptides, oligonucleotides, peptidic nucleic acids, drugs, etc.) [Prochiantz, A., 1996]. Taking the “Iliad” a tale from the Greek mythologies as an example these cell-penetrating peptides (CPP) were also termed ‘Trojan’ peptides. Most of them are water-soluble peptides with a low lytic activity that can be used as vectors for cellular internalization of hydrophilic biomolecules and drugs [Lindgren, M., et al., 2000, Stephens, D.J., & Pepperkok, R. 2001]. Despite its broad application field, the internalization mechanism of the penetratin peptide has not been totally unraveled yet. It appears that a receptor or a transporter protein is not needed, as retro-enantio and retro-inverso analogs of penetratin are also internalized [Derossi, D., et al., 1996]. Presumably, cellular internalization of the penetratin peptide occurs via a direct interaction with the cell membrane [Prochiantz, A., 1996)].
Table 1 contains a list of peptides that have been investigated for their ability to penetrate the cell. The potential to inhibit specific mRNA export pathways using cell-permeable peptides is shown in figure 1. Cell-penetrating peptide inhibitors are inhibiting their target proteins rapidly. In that sense they could be superior to other “functional knockout” approaches such as RNA interference (RNAi). RNAi prevents translation of a protein by destroying its mRNA; however, a long-lived protein will continue to be active long after its synthesis has stopped. The more rapidly a peptide affects RNA export, the more likely it is that the peptide directly (rather than indirectly) inhibits export of the target RNA. It is conceivable that designer inhibitory peptides based on the sequences of cell-penetrating peptides have the potential to allow for the generation of a mammalian cell tool kit that could parallel the temperature-sensitive mutant collection available to yeast geneticists [Moore and Rosbash, 2001].
Table 1: Cell-penetrating peptides that translocate into the cell (Futaki, S. 2005)
Residues highlighted in dark bold blue indicate a positive charge where as the ones highlighted in bold red indicate negative charges.
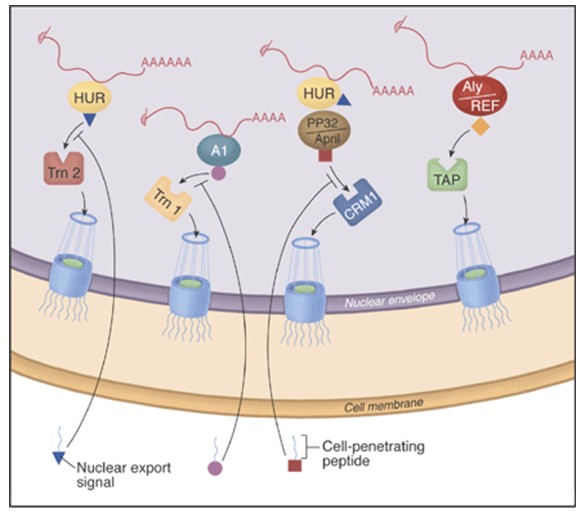
Figure 1: The potential to inhibit specific mRNA export pathways with cell-permeable peptides. The major route of mRNA export from the nucleus may depend on interactions between the mRNA adaptor protein Aly/REF and the export receptor heterodimer TAP:p15, which in turn interacts with the nuclear pore complex. New inhibitors that couple cell-penetrating peptides with specific nuclear export signals reveal that some mRNAs use other adaptors and receptors. For example, there are two nuclear export pathways for c-fos mRNA. One pathway involves the adaptor protein HuR and the export receptor Trn2, whereas the other involves HuR, its two ligands (pp32 and APRIL) and the export receptor CRM1. [Melissa J. Moore and Michael Rosbash in SCIENCE VOL 294 p. 1841, 2001].
3D structures
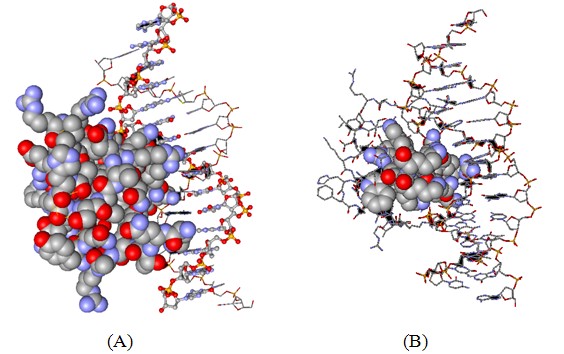
Figure 2: Three-dimensional structure of the antennapedia homeodomain protein-DNA complex. (Protein Data Bank accession code 9ant).
A: The protein is shown as the space-fill model whereas the DNA is displayed in ball-and-stick (one DNA strand) and stick mode (second DNA strand).
B: Only the peptide corresponding to penetratin peptide is now displayed in the space-filling mode all other atoms are in the stick mode. It is apparent that the peptide is in close contact with the DNA molecule apparently binding to the major groove of the helix.
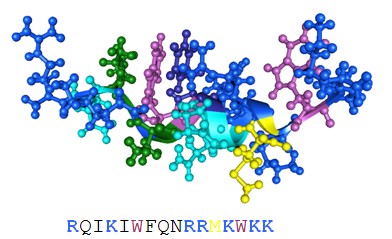
Figure 3: Three-dimensional solution structure of penetratin. (Protein Data Bank accession code 1kz0).
Amino acid side chains of basic amino acid residues are highlighted in blue. The tryptophans are shown in purple. The primary structure of the peptides is also provided. All pictures were generated with the 3D mol viewer of Vector NT (version 6, Invitrogen, USA).
References
Derossi, D., Calvet, S., Trembleau, A., Brunissen, A., Chassaing, G. & Prochiantz, A. (1996) Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271, 18188–18193.
Derossi, D., Chassaing, G. & Prochiantz, A. (1998) Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 8, 84–87.
Dunican, D.J. & Doherty, P. (2001) Designing cell-permeant phosphopeptides to modulate intracellular signaling pathways. Biopolymers 60, 45–60.
Fischer, P.M., Zhelev, N.Z., Wang, S., Melville, J.E., Fahraeus, R. & Lane, D.P. (2000) Structure-activity relationship of truncated and substituted analogues of the intracellular delivery vector Penetratin. J. Pept. Res. 55, 163–172.
Futaki, S. (2002) Arginine-rich peptides: potential for intracellular delivery of macromolecules and the mystery of the translocation mechanisms. Int. J. Pharm. 245, 1–7.
Futaki, S. (2002) Membrane-permeable arginine-rich peptides and the translocation mechanisms. Adv. Drug Del. Reviews 57, 547-558.
Joliot, A., Pernelle, C., Deagostini-Bazin, H. & Prochiantz, A. (1991) Antennapedia homeobox peptide regulates neural morphogenesis. Proc. Natl Acad. Sci. USA 88, 1864–1868.
Lindgren, M., Hallbrink, M., Prochiantz, A. & Langel, U. Cell-penetrating peptides. (2000) Trends Pharmacol. Sci. 21, 99–103.
Moore, M.J., and Rosbash, M., (2001) Cell biology. TAPing into mRNA export. Science 294, 1841-2.
Pooga,M., Halbrink, M, Zorko, M, Langel, U., (1998) Cell Penetration by Transportan. FASEB Journal,12;67-77.
Pooga M, Land T, Bartfai T, Langel U. (2001) PNA oligomers as tools for specific modulation of gene expression. Biomol Eng. 2001 Jun;17(6):183-92.Prochiantz, A. (1996) Getting hydrophilic compounds into cells: lessons from homeopeptides. Curr. Opin. Neurobiol. 6, 629–634.
Prochiantz, A. (1996) Getting hydrophilic compounds into cells: lessons from homeopeptides. Curr. Opin. Neurobiol. 6, 629–634.
Saalik P, Elmquist A, Hansen M, Padari K, Saar K, Viht K, Langel U, Pooga M. (2004) Protein cargo delivery properties of cell-penetrating peptides. A comparative study. Bioconjug Chem. Nov-Dec;15(6):1246-53.
Stephens, D.J., & Pepperkok, R. The many ways to cross the plasma membrane. (2001) Proc. Natl Acad. Sci. USA 98, 4295–4298
Thorén, P.E., Persson, D., Isakson, P., Goksör, M., Önfelt, A. & Nordén, B. (2003) Uptake of analogs of penetratin, Tat (48–60) and oligoarginine in live cells. Bioch. Biophys. Res. Comm. 307, 100–107.
Zorko, M. and Langel, U., (2005) Cell-penetrating peptides: mechanism and kinetics of cargo delivery. Adv. Drug Del. Reviews 57, 529-545.