Molecular Beacons
Molecular beacons are
oligonucleotide probes that report the presence of specific nucleic acids in homogeneous solutions
. They are useful in situations where it is either not possible or desirable to isolate the probe-target hybrids from an excess of the hybridization probes, such as in real time monitoring of polymerase chain reactions in sealed tubes or when detecting RNA molecules within living cells. Molecular beacons are single-stranded oligonucleotides that possess a hairpin structure. These hairpin shaped molecules contain an internally quenched fluorophore whose fluorescence is restored when they bind to a target nucleic acid as illustrated in figure 1. They are designed in such a way that the loop portion of the molecule is a probe sequence complementary to a target nucleic acid molecule. The stem is formed by the annealing of complementary arm sequences on the ends of the probe sequence. A fluorescent moiety is attached to the end of one arm and a quenching moiety is attached to the end of the other arm. The stem keeps these two moieties in close proximity to each other, causing the fluorescence of the fluorophore to be quenched by energy transfer. Since the quencher moiety is a non-fluorescent chromophore and emits the energy that it receives from the fluorophore as heat, the probe is unable to fluoresce. When the probe encounters a target molecule, it forms a hybrid that is longer and more stable than the stem and its rigidity and length preclude the simultaneous existence of the stem hybrid. Thus, the molecular beacon undergoes a spontaneous conformational reorganization that forces the stem apart, and causes the fluorophore and the quencher to move away from each other, leading to the restoration of fluorescence.
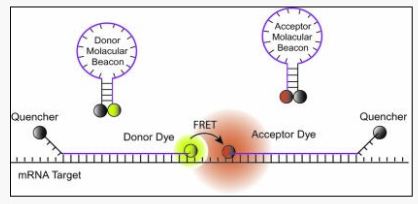
Figure 1: Operation of molecular beacons: On their own, these molecules are non-fluorescent, because the stem hybrid keeps the fluorophore close to the quencher. When the probe sequence in the loop hybridizes to its target, forming a rigid double helix, a conformational reorganization occurs that separates the quencher from the fluorophore, restoring fluorescence.
In order to detect multiple targets in the same solution, molecular beacons can be made in many different colors utilizing a broad range of fluorophores. DABCYL, a non-fluorescent chromophore, serves as the universal quencher for any fluorophore in molecular beacons. Owing to their stem, the recognition of targets by molecular beacons is so specific that single-nucleotide differences can be readily detected.
Steps needed for a real-time based PCR assay using molecular beacons
- Target Design
- Primer Design
- Optimization of the amplification reaction conditions
- Molecular Beacon Design
- Molecular Beacon Synthesis and Characterization
Parameters for maximal potential of real-time PCR
- Optimal amplification conditions
- Optimal design so that the beacons are able to hybridize with their target
- Optimal efficiency and accuracy of real-time PCR
- Assay specific optimization
- Optimized beacon to detect the selected specific target sequence
Molecular Beacon Design
There are two independent features to control in the design of the molecular beacon probe and the PCR primers themselves should have been optimized in a regular PCR to ensure that the PCR reaction works well. Assuming the melting temperature of the primers are ~55 °C. The stem and the target loop sequence. Design the probe sequence and see that there is minimal secondary structures, loop formation and dimers and the TM is ~5 °C higher than the PCR primer annealing temp. For a good guideline keep it at ~60 °C. Add the stem sequence of 5-7 bp. The TM of the stem itself will be ~ 60-70 °C.
General guidelines for Molecular Beacon design at Bio-Synthesis are as follows:
- Design regular 18 to 24mer PCR primers for amplification with a Tm around 55 °C. The optimal amplified fragment should be between 100 to 300 base pairs. Perform PCR and optimize reaction conditions as needed. A good desing hould result in a clean amplification product visible on ethidium bromide stained gels.
- Design target probe sequence with a Tm ~8 to 10 °C higher than the annealing temperature of the PCR primers used. Example 60 to 65 °C: The probe should be designed near the center of the amplified fragment by avoiding sequence stretches that can form a secondary structure. [Taqman probes are designed with ~ 5 to 10 bases near the primer of the same strand]
- Add the stem using 5 to 7 base pairs stem sequence with a GC content of 70-80%. Avoid a G at the 5‟ end next to the fluorophore. G‟s seem to have a quenching effect. Hairpin Stem TM should be 7 to 10 °C higher than the PCR annealing temperature. Example 65 to 70 °C.
Factors that influence that the molecular beacon remains closed in absence of target are
- Sequence length of the arm region
- Nature of sequence of the arm region
- G-C content of the resulting stem
- The Tm should be 7 to 10 °C higher than the detection temperature which is usually the annealing temperature of the PCR
Caution: When adding the stem sequence avoid sequences that can form secondary structures by hybridizing with the loop sequence. Try several variations of the stem sequence to avoid the formation of secondary structures with the loop sequence.
Hairpin Tm: The Hairpin stem TM is based on free energy stabilization and folding, the following is a good guideline.
Melting temperatures of G-C rich stem sequences
5 bp = 55 °C to 60 °C
6 bp = 60 °C to 65 °C
7 bp = 65 °C to 70 °C
Reference
Tyagi S, Kramer FR. Molecular beacons: probes that fluoresce upon hybridization, Nature Biotechnology 1996; 14: 303-308.
Tyagi S, Bratu DP, Kramer FR. Multicolor molecular beacons for allele discrimination, Nature Biotechnology 1998; 16: 49-53.
Marras SAE, Kramer FR, and Tyagi S (2003) Genotyping single nucleotide polymorphisms with molecular beacons. In Kwok, P. Y. (ed.), Single nucleotide polymorphisms: methods and protocols. The Humana Press Inc., Totowa, NJ, Vol. 212, pp. 111-128. PDF
Vet, J.A.M. and Marras, S.A.E. (2004) Design and optimization of molecular beacon real-time polymerase chain reaction assays. In Herdewijn, P. (ed.), Oligonucleotide synthesis: Methods and Applications. Humana Press, Totowa, NJ, Vol. 288, pp. 273-290.