Modified oligonucleotides allowed researchers to gain insight into conformational, mechanistic and sterochemical aspects of a variety of biological reactions and processes. Using 2'-Fluoroinosine instead of inosine increases the stability of chimeric oligonucleotides. The incorporation of inosine and 2'-fluoroinosine in primers and probes at specific locations modulates selectivity. The phosphoramidite 2'-fluoro-inosine-CE phosphoramidite allows the synthesis of oligonucleotides containing 2'-fluoroinosine (2F-I).
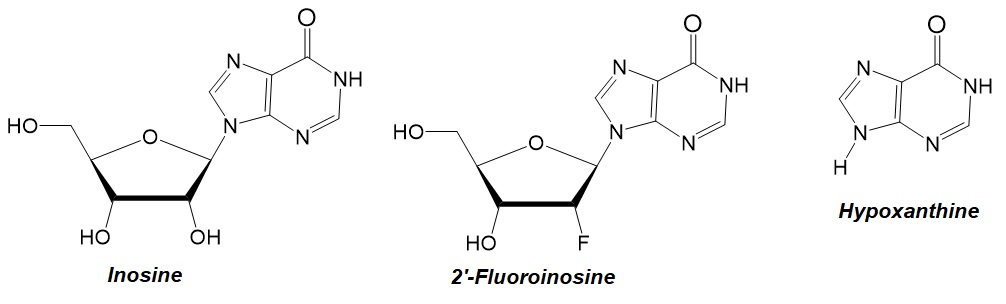
Figure 1: Chemical structures of inosine, 2'-fluoroinosine, and hypoxanthine.
Synthetic oligonucleotides containing inosine have been extensively used as hybridization probes to screen cDNA or genomic libraries for genes encoding proteins whose amino acid sequence is only partially known. Inosine-containing oligonucleotides enabled the cloning of many genes from genomic and cDNA libraries of high complexity. Inosine interacts with each of the principal nucleic acid bases through two hydrogen bonds. Interactions in order of decreasing stability are I-C > I-A > I-T ≈ I-G.
Microbial studies routinely use universal primers. Usually, this type of research starts with the extraction of total DNA from bacterial samples, followed by PCR amplification of small-subunit ribosomal RNA genes. Designing degenerate primers with varying options of nucleotides at several positions in the internal primer sequence allows improved amplification of related sequences of 16S rRNA genes from different microorganisms. This approach allows the amplification of a variety of related sequences. Often inosine is used to achieve a fourfold degeneracy for a given location. Using inosine at the 3’-terminal ends of 16S ribosomal RNA (rRNA) gene universal primers allowed studying complex microbial communities by PCR. For example, Ben-Dov et al. replaced the 3’-terminal nucleic acid with inosine in universal 16S rRNA primers to study complex microbial communities to expand the observed diversity of a microbial community under study.
The introduction of 2'-fluoro RNA can modulate the selectivity oligonucleotides, as was found to be the case for antisense oligonucleotides (ASOs). The electronegative fluorine atom produces local changes in the conformation of the nucleotide furanose ring. The incorporation of 2'-fluoro-inosine affects the biological properties of the modified oligonucleotide. Structural studies performed by Martin-Pintado showed that oligonucleotides containing alternating and contiguous tracts of 2′F‐RNA and 2′F‐ANA nucleotides reveal that nonconventional FC-H⋅⋅⋅O hydrogen bonds have a strong stabilizing effect on 2′‐fluorinated duplexes. Hence, replacing inosine with 2'-fluoroinosine in oligonucleotides increases the stability of the duplex formed with complementary oligonucleotides.
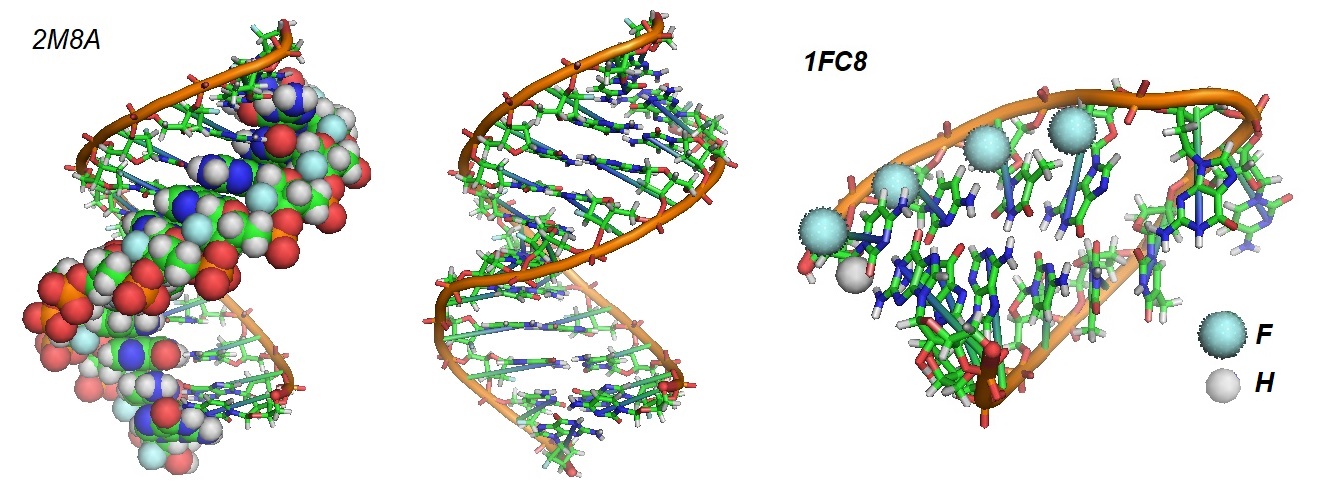
Figure 2: Molecular structures of 2'-fluoro-substituted oligonucleotides. A duplex with 2'-fluoro-bases is shown to the left and middle of the figure (Martin-Pintado et al. 2013). The picture to the right shows the structure for a 2'-deoxy-2'-fluoro-D-arabinose nucleic acid (2'F-ANA)/RNA duplex (Trempe et al. 2001). The (2'F-ANA)/RNA duplex exhibits structural parameters between those of A-form and B-form duplexes and similar to those of DNA/RNA duplexes. The enhanced stability of the duplex is relevant to the design of new antisense drugs based on sugar-modified nucleic acids.
Reference
Ben-Dov E, Shapiro OH, Siboni N, Kushmaro A. Advantage of using inosine at the 3' termini of 16S rRNA gene universal primers for the study of microbial diversity. Appl Environ Microbiol. 2006 Nov;72(11):6902-6. [PMC]
Green & Sambrook; Molecular Cloning. A Laboratory Manual. 4th Edition. 2012, Cold Spring Harbor Laboratory Press.
Martin-Pintado N., Deleavey G.F., Portella G., Campos-Olivas R., Orozco M., Damha M.J., Gonzalez C., Backbone F.C.-H. O hydrogen bonds in 2′F-substituted nucleic acids. Angew. Chem. Int. Ed. 2013;52:12065–12068. [PubMed]
Pallan PS, Greene EM, Jicman PA, Pandey RK, Manoharan M, Rozners E, Egli M.; Unexpected origins of the enhanced pairing affinity of 2'-fluoro-modified RNA. Nucleic Acids Res. (2011) 39 p.3482-95. [PMC]
J F Trempe , C J Wilds, A Y Denisov, R T Pon, M J Damha, K Gehring ; NMR solution structure of an oligonucleotide hairpin with a 2'F-ANA/RNA stem: implications for RNase H specificity toward DNA/RNA hybrid duplexes. J Am Chem Soc. 2001 May 30;123(21):4896-903. [PubMed]
Bio-Synthesis provides a full spectrum of high-quality custom oligo modification services by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides containing backbone, base, sugar, and internucleotide linkages. Bio-Synthesis specializes in synthesizing complex modified oligonucleotides using phosphodiester backbone, purine, pyrimidine heterocyclic bases, and sugar modified nucleotides such as our patented 3rd generation Bridged Nucleic Acids.
.png)
---...---