Therapeutic oligonucleotides affect RNA-processing reactions such as mRNA degradation, pre-mRNA splicing, or translation. Several therapeutic oligonucleotides are now approved for clinical correction of genetic mutations.
Molecular Action Platforms For Therapeutic Oligonucleotides
Three types of molecular actions have emerged as significant platforms for therapeutic oligonucleotides:
[1] RNase H-dependent degradation: short chimeric antisense oligonucleotides called gapmers enable the correction of splicing defects.
[2] RNA-interference: Known as RNAi or siRNA, siRNA oligonucleotides mediate the degradation of the transcript to silence genes.
[3] Splicing modulation: Steric-blocking antisense oligonucleotides allow modulation of aberrant splicing events.
Therapeutic Oligonucleotides are Modified
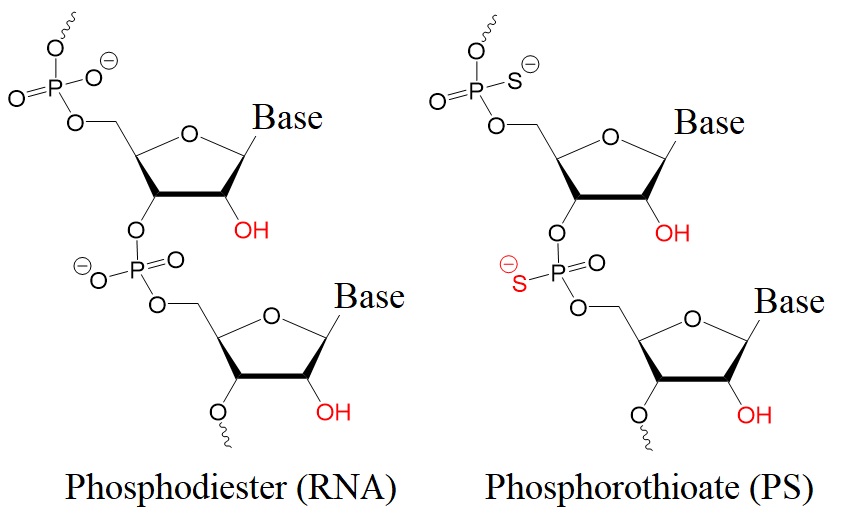
To enhance therapeutic oligonucleotides' stability and efficiency, chemical modifications in their phosphate backbone and sugar rings are usually used. Most common versions of antisense oligonucleotides (AONs) contain phosphorothioate backbones (PS). Here, a sulfur atom replaces one oxygen atom in the phosphodiester group. Also, a 2′-O-methyl group (2′-OMe) is used at the 2′-position in the ribose sugar.
However, other modifications are also investigated for their ability to enhance the function of therapeutic oligonucleotides investigated.
The 2'-O-[2-(methylamino)-2-oxoethyl] (2'-O-NMA) modification is a new chemical modification that enhances the stability, specificity, and therapeutic potential of RNA molecules.
|
|
|
|
Figure 1: Chemical structures of a phosphodiester (RNA) and a phosphorothioate (PS).
|
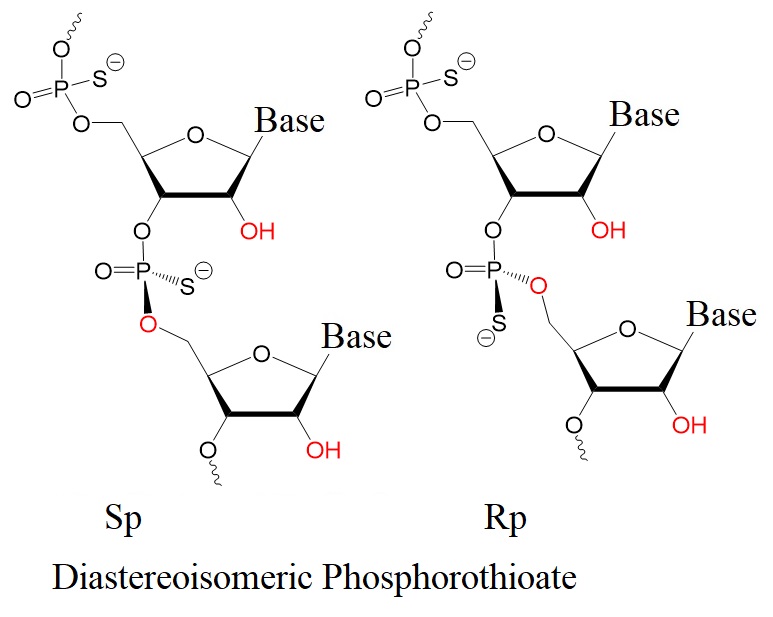
Figure 2: Rp and Sp diastereoisomers of phosphorothioates.
|
The 2'-O-NMA modification, also known as the 2'-O-[2-(methylamino)-2-oxoethyl] group, is a chemical modification applied to nucleic acids, particularly RNA molecules. This modification involves introducing the 2'-O-NMA group at the 2'-position of the ribose sugar in RNA. This modification enhances the stability, specificity, and therapeutic potential of RNA molecules.
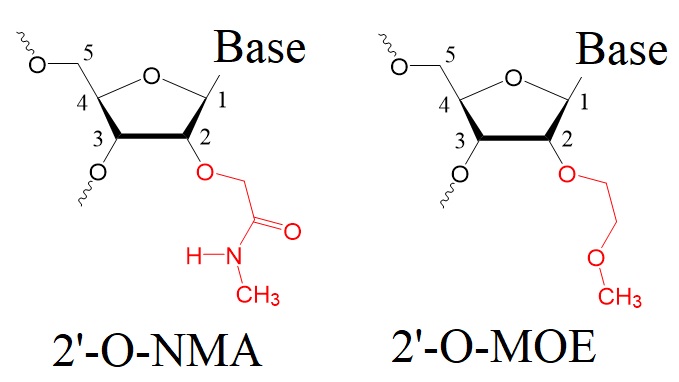
Figure 3: Structures of 2'-O-[2-(methylamino)-2-oxoethyl] group modified (2’-O-NMA) and 2’-O-methoxyethyl (2’-O-MOE) modified oligonucleotides.
2'-O-NMA modified oligonucleotides enable the development of RNA mimics for antisense therapeutics. Antisense therapeutics use modified oligonucleotides to target specific RNA sequences to enable modulation of gene expression. RNA mimics imitate the function of natural RNA molecules, allowing for selective targeting and regulation of gene expression.
Incorporating 2'-O-NMA into antisense RNA oligonucleotides provides several advantages over unmodified RNA molecules. Introducing the 2'-O-NMA modification enhances the stability and resistance to degradation of RNA mimics in biological systems. This modification improves the pharmacokinetic properties of the molecule, allowing for increased cellular uptake and prolonged half-life.
Also, 2'-O-NMA enhances the affinity and specificity of RNA mimics for their target sequences. The modification strengthens the binding between the RNA mimic and its complementary RNA sequence, resulting in improved target recognition and increased potency of the therapeutic effect. The increased affinity allows lower doses of the therapeutic RNA oligonucleotide for administration while achieving the desired medicinal outcome.
Antisense oligonucleotides can designed with 2'-O-NMA modification to develop RNA-based drugs for various diseases. This modification offers several advantages over unmodified RNA molecules:
1. Increased stability: Adding the 2'-O-NMA modification improves the stability of RNA molecules. The modification protects the RNA from degradation by cellular ribonucleases, which can rapidly break down unmodified RNA. This increased stability enables the RNA molecule to remain intact for longer, allowing it to perform its intended function more effectively.
2. Enhanced specificity: The 2'-O-NMA modification also improves the specificity of RNA molecules. It reduces off-target effects. As a result, the modified RNA is less likely to interact with unintended cellular components or trigger unwanted immune responses. This enhanced specificity is crucial for therapeutic applications, as it increases the safety and efficacy of RNA-based drugs.
3. Improved pharmacokinetics: Pharmacokinetics refers to how drugs are absorbed, distributed, metabolized, and eliminated by the body. The 2'-O-NMA modification can positively influence the pharmacokinetic properties of RNA molecules. It can increase their resistance to enzymatic degradation and improve cellular uptake, distribution, and retention. This results in better bioavailability and therapeutic outcomes.
4. Reduced immunogenicity: The immune system recognizes specific RNA molecules as foreign and mounts an immune response against them, limiting the effectiveness of RNA-based therapies. The 2'-O-NMA modification helps to reduce the immunogenicity of RNA by decreasing its recognition by the immune system. By minimizing immune responses, the modified RNA has a higher chance of successfully reaching its target and exerting its therapeutic effect.
Design of 2'-O-NMA-based RNA mimics
The design and synthesis of 2'-O-NMA-based RNA mimics for antisense therapeutics require careful consideration of several factors.
[1] Carefully identify the specific target RNA sequence.
[2] Design complementary sequences so that they can bind to the target with high affinity and specificity. Additionally.
[3] Optimize the overall secondary structure and stability of the RNA mimic to ensure efficient delivery and intracellular activity.
Overall, the 2'-O-NMA modification is a valuable tool in RNA-based therapeutics. It improves the stability, specificity, pharmacokinetics, and immunogenicity profile of RNA molecules, enhancing their potential as therapeutic agents. Ongoing research and development efforts continue to explore the full potential of this modification and its applications in treating various diseases, including genetic disorders, viral infections, and cancer. Continued research and development in this field hold promise for the advancement of RNA-based therapeutics and their application in treating various diseases.
Reference
Adachi H, Hengesbach M, Yu Y-T, Morais P. From Antisense RNA to RNA Modification: Therapeutic Potential of RNA-Based Technologies. Biomedicines. 2021; 9(5):550. [PMC]
Egli M, Pallan PS. Crystallographic studies of chemically modified nucleic acids: a backward glance. Chem Biodivers. 2010 Jan;7(1):60-89. [PMC]
Egli M, Manoharan M. Re-Engineering RNA Molecules into Therapeutic Agents. Acc Chem Res. 2019 Apr 16;52(4):1036-1047. [ACS]
Pattanayek R, Sethaphong L, Pan C, Prhavc M, Prakash TP, Manoharan M, Egli M. Structural rationalization of a large difference in RNA affinity despite a small difference in chemistry between two 2'-O-modified nucleic acid analogues. J Am Chem Soc. 2004 Nov 24;126(46):15006-7. [ACS]
Prakash TP, Kawasaki AM, Wancewicz EV, Shen L, Monia BP, Ross BS, Bhat B, Manoharan M. Comparing in vitro and in vivo activity of 2'-O-[2-(methylamino)-2-oxoethyl]- and 2'-O-methoxyethyl-modified antisense oligonucleotides. J Med Chem. 2008 May 8;51(9):2766-76. [PubMed]
Sheng L, Rigo F, Bennett CF, Krainer AR, Hua Y. Comparison of the efficacy of MOE and PMO modifications of systemic antisense oligonucleotides in a severe SMA mouse model. Nucleic Acids Res. 2020 Apr 6;48(6):2853-2865. [PMC]
Ziemkiewicz K, Warminski M, Wojcik R, Kowalska J, Jemielity J. Quick Access to Nucleobase-Modified Phosphoramidites for the Synthesis of Oligoribonucleotides Containing Post-Transcriptional Modifications and Epitranscriptomic Marks. J Org Chem. 2022 Aug 5;87(15):10333-10348. [PMC]
---...---
Bio-Synthesis provides a full spectrum of bio-conjugation services including high quality custom oligonucleotide modification services, back-bone modifications, conjugation to fatty acids and lipids, cholesterol, tocopherol, peptides as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---