Fluorescent probes used as biological indicators can provide useful information about physiological changes in cells. Rationally designed probes containing dyes that respond linearly to the target molecule or ion allow quantitation of the target molecule or ion independent of the dye, the instrumentation as well as the thickness of the sample. Many molecular indicators based on intermolecular Fluorescence Resonance Energy Transfer (FRET), as well as Bioluminescence Resonance Energy Transfer (BRET), were developed in the last decades. Among them are chemically and genetically encoded FRET-based sensor proteins or peptides that have significantly enhanced our understanding of the intracellular functions of Zn2+.
Zinc is an essential structural component of many DNA binding proteins that plays a significant role in cellular signaling including in mitosis and the suppression of apoptosis in cells. Furthermore, zinc is essential for the function of the immune system. Both, the innate and adaptive immune system involve regulating intracellular signaling pathways based on zinc ions. The expression and action of zinc “importers” (ZIP 1–14), zinc “exporters” (ZnT 1–10), and zinc-binding proteins control the role of zinc in cells.
Hence, zinc is an essential micronutrient, and the leading cause of zinc deficiency is malnutrition, for example, cell-mediated immune dysfunctions.
In 1996, Godwin and Berg showed that a zinc finger consensus peptide, CP, can be used for the design of a cellular zinc probe. Since zinc finger peptides bind zinc tightly and selectively, they provide an ideal framework for selective zinc probes
Figure 1 illustrates the consensus peptide sequence and its location within the protein fold of a zinc finger protein used for the design of the probe.
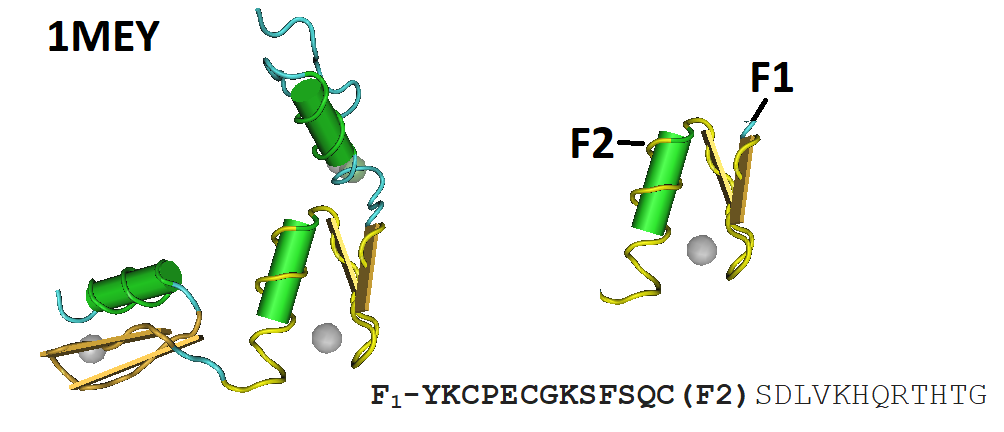
Figure 1: The crystal structure of a complex between a protein with three consensus-sequence-based zinc finger domains and an oligonucleotide corresponding to a favorable DNA binding site (1MEY) is illustrated here. The structure of this sequence-specific DNA binding protein based on tandem arrays of Cys2His2 zinc finger domains revealed the modular interactions and structural adaptations that compensate for differences in contact residue side-chain lengths.
Peptide-based FRET-probes are synthesized using Fmoc-chemistry based solid phase peptide synthesis. For this particular probe, the dyes fluorescein (495, 521 nm) and lissamine (578, 596 nm) are covalently conjugated to the peptide. The fluorophore fluorescein (F2) is the donor and lissamine (F1) is the acceptor molecule of the peptide probe. In the absence of zinc (II) the peptide is unfolded and the dyes are relatively far apart. Therefore, the amount of intermolecular energy transfer between the chromophores is small. Upon binding of the zinc ion, the peptide folds, and the fluorophores are brought closer together which now increases the amount of intermolecular interaction and energy transfer. The peptide probe takes advantage of the highly efficient, specific, and tunable zinc binding characteristics of this peptide-based FRET-probe.
Reference
Aper SJ, Dierickx P, Merkx M. Dual Readout BRET/FRET Sensors for Measuring Intracellular Zinc. ACS Chem Biol. 2016 Oct 21;11(10):2854-2864. doi: 10.1021/acschembio.6b00453. Epub 2016 Aug 22. PubMed PMID: 27547982; PubMed Central PMCID: PMC5080634.
Gammoh NZ, Rink L. Zinc in Infection and Inflammation. Nutrients. 2017 Jun 17;9(6):624. doi: 10.3390/nu9060624. PubMed PMID: 28629136; PubMed Central PMCID: PMC5490603.
Hilary Arnold Godwin, and Jeremy M. Berg; A Fluorescent Zinc Probe Based on Metal-Induced Peptide Folding. J. Am. Chem. Soc., 1996, 118 (27), pp 6514–6515. DOI: 10.1021/ja961184d.
Kim CA, Berg JM.; A 2.2 A resolution crystal structure of a designed zinc finger protein bound to DNA. Nat Struct Biol. 1996 Nov;3(11):940-5.
Wessels I, Maywald M, Rink L. Zinc as a Gatekeeper of Immune Function. Nutrients. 2017 Nov 25;9(12):1286. doi: 10.3390/nu9121286. PubMed PMID: 29186856; PubMed Central PMCID: PMC5748737. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5748737/
---…---