Bio-orthogonal reactions allow the tagging of proteins, carbohydrates, nucleic acids, and oligonucleotides (DNA and RNA) with reporter molecules, the conjugation of molecular building blocks to each other, and other selected biomolecules of interest as well.
The Staudinger ligation is a bio-orthogonal reaction helpful in studying biomolecules. Staudinger ligation allows the formation of a native amide bond between a tagging group and a biomolecule. Staudinger ligation enables coupling azides to phosphines to form an amide bond. The German chemists Hermann Staudinger and Jules Meyer first described the Staudinger reaction in the early 20th century, which has since become a standard tool in chemical biology.
The general form of the reaction is shown here:
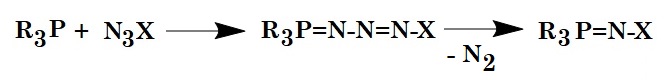
Figure 1: Classical Staudinger Reaction.
During the Staudinger reaction, triphenylphosphines react with azides forming an intermediate iminophosphorane with the release of nitrogen gas. In the presence of water molecules, the intermediate breaks down into a triphenylphosphine oxide and a primary amine.
In 2000, Saxon and Bertozzi introduced a modified Staudinger reaction that enabled a coupling reaction with biomolecules, now known as Staudinger ligation enabling the conjugation of biomolecules in living animals.
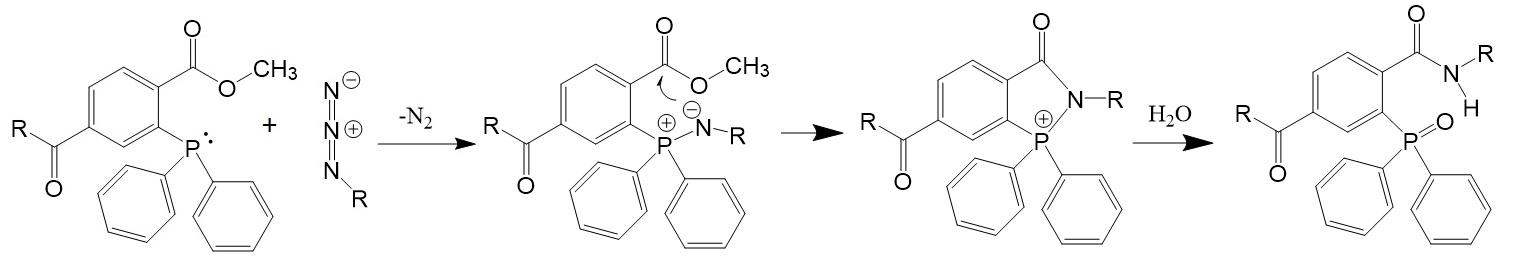
Figure 2: Modified Staudinger reaction (Saxon & Bertozzi. Science 2000, 287, 2007-2010; Hermanson G.T., Bioconjugate Techniques. 3rd Editon. Academic Press 2013).
Twenty-two years later, in 2022, the Nobel Prize in chemistry was awarded to Carolyn R. Bertozzi, Morten Meldal and K. Barry Sharpless for their development of click chemistry and bio-orthogonal chemistry.
Staudinger ligation is particularly useful in chemical biology because it allows site-specific modifications of biological molecules, such as oligonucleotides, proteins, peptides and nucleic acids.
The Staudinger ligation reaction utilizes the introducing of an azide group into a target molecule by genetic or chemical means and then reacting it with a phosphine-containing reagent to form a covalent bond between the target molecule and the reagent. The reaction allows labeling or modifying specific sites within a complex biological system for the study of biological processes in situ. The azide group can be incorporated into amino acids, lipids, oligonucleotides (DNA and RNA), and sugars to label these molecules in vivo or in vivo using phosphine probes.
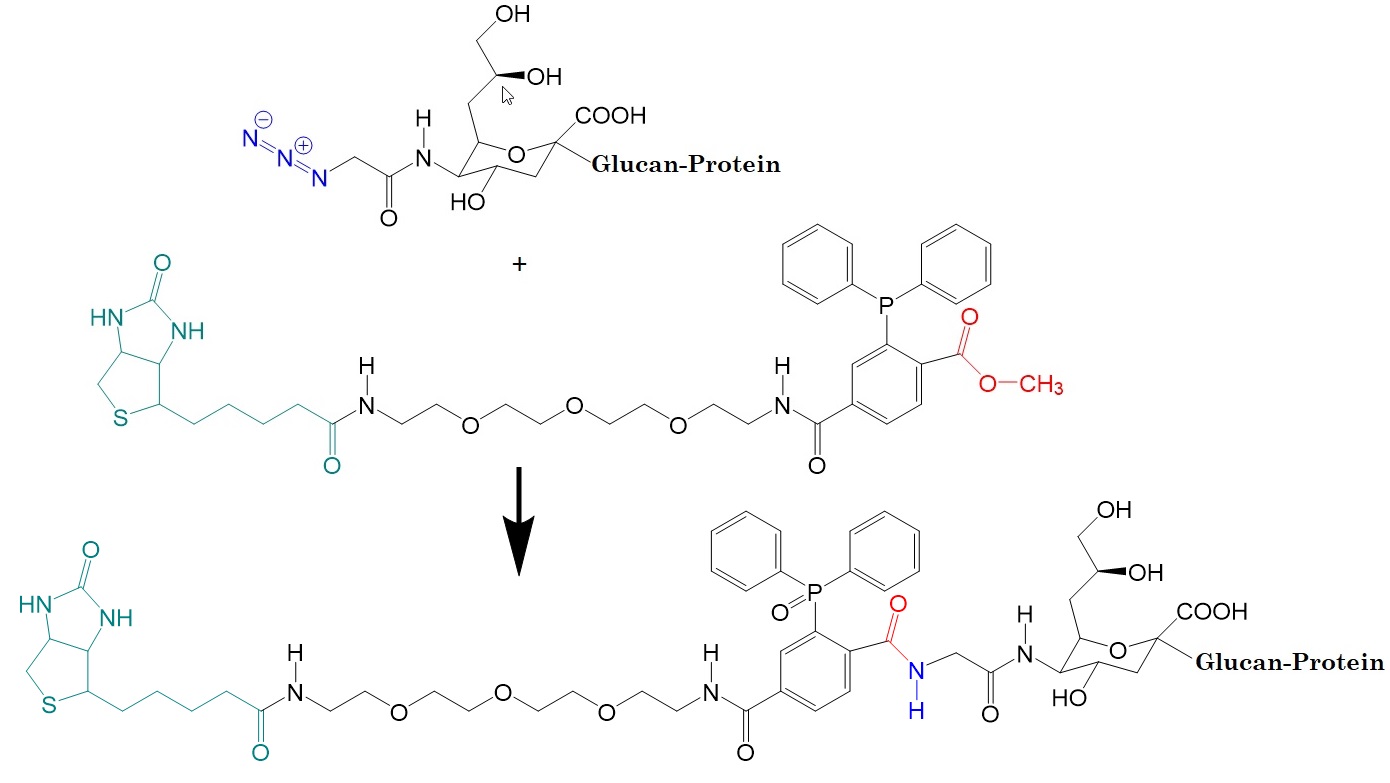
Figure 3: Example of a Staudinger ligation reaction utilizing a biotin-phosphine tag for specific labeling of azido-sialic acid derivatives in vitro and in vivo.
In the traceless variant of the Staudinger ligation, a modified phosphine reagent containing a cleavable linker allows the removal of the phosphine after the reaction.
The traceless Staudinger ligation eliminates the need for a separate step to remove the phosphine from the product, resulting in a more efficient and practical valuable approach for biological applications.
Reference
Click chemistry and biorthogonal chemistry. Scientific background. [pdf]
Hermanson G.T., Bioconjugate Techniques. 3rd Editon. Academic Press 2013.
Kitoun C, Fonvielle M, Sakkas N, Lefresne M, Djago F, Blancart Remaury Q, Poinot P, Arthur M, Etheve-Quelquejeu M, Iannazzo L. Phosphine-Mediated Bioconjugation of the 3'-End of RNA. Org Lett. 2020 Oct 16;22(20):8034-8038. [ACS]
Kitoun C, Fonvielle M, Arthur M, Etheve-Quelquejeu M, Iannazzo L. Traceless Staudinger Ligation for Bioconjugation of RNA. Curr Protoc. 2021 Feb;1(2):e42. [CURRENT PROTOCOLS]
Nobel Prize in Chemistry 2022
Saxon & Bertozzi. Cell surface engineering by a modified Staudinger reaction Science 2000, 287, 2007-2010. [Science]
Sletten EM, Bertozzi CR. From mechanism to mouse: a tale of two bioorthogonal reactions. Acc Chem Res. 2011 Sep 20;44(9):666-76. [PMC]
Staudinger Reaction [Wiki]
---...---
Bio-Synthesis provides a full spectrum of bio-conjugation services including high quality custom oligonucleotide modification services, back-bone modifications, conjugation to fatty acids and lipids, cholesterol, tocopherol, peptides as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---