The number of efficient delivery systems for therapeutic molecules into cells still needs to be increased. SiRNA introduced into cells drives the RNA interference (RNAi) process. Because of the high molecular weight and the negative charge of the phosphate backbone, siRNA molecules cannot readily cross the cell membrane, presently limiting the clinical and therapeutic value of siRNAs.
Research projects are ongoing to find the best approaches for delivering siRNAs through the cell membrane into the cytoplasm.
Cell-penetrating peptides (CPPs) can trigger the movement of molecules across cell membranes, including mitochondrial and nuclear membranes, without destroying them. These short peptides can translocate and transport cargo molecules into the intracellular space by crossing biological membranes. Complexed or conjugated to siRNA oligonucleotides, CPPs may offer the design of efficient cell delivery systems for enhanced delivery of oligonucleotide-based therapeutics.
The plasma membrane protects cells against the entrance of external molecules. The plasma membrane separates the intracellular cytosol from the extracellular environment to maintain cellular homeostasis. This barrier makes accessing cells difficult for extracellular hydrophilic molecules such as peptides, proteins, and nucleic acids.
A variety of strategies are applied to overcome this permeability issue. Historically, viral and non-viral approaches have been the primary delivery strategies. Viral vectors utilized for gene therapy allow the delivery of genetic material across the cell membrane with high efficacy.
Besides viral delivery, electroporation, encapsulation, and association of drugs with lipids, peptides, polymers, nanotubes, liposomes, micelles, and dendrimers have been investigated for their ability to deliver therapeutic oligonucleotides into cells with high efficiency.
Still, the use of vectors encounters several challenges, such as limited cargo-carrying capacity, low delivery efficiency, the risk of mutation, high cytotoxicity, and lack of target specificity. Also, viral vectors are incompatible with all kinds of nucleic acid-based molecules, for example, the delivery of short synthetic Oligonucleotides.
Also, scientists must overcome environmental and enzymatic degradation to develop an efficient therapeutic siRNA delivery system.
siRNAs are double strand (ds) RNAs, ~19–23 base pairs with a characteristic 3′- overhang that facilitates the recognition by the RNAi enzymatic machinery.
Usually, in RNAi, Dicer cleaves long dsRNAs into siRNAs. siRNAs then bind to the RNA-induced silencing complex (RISC), which unwinds the siRNAs and removes the sense strand for degradation by the cell’s nucleases. The antisense strand targets specific mRNA sequences, directs them to the RISC, and anneals with the complementary base pair. Finally, the targeted mRNA is rapidly degraded, resulting in a decrease in protein expression.
The following figures show structural models of siRNA gleaned from crystal structures of siRNA protein complexes.
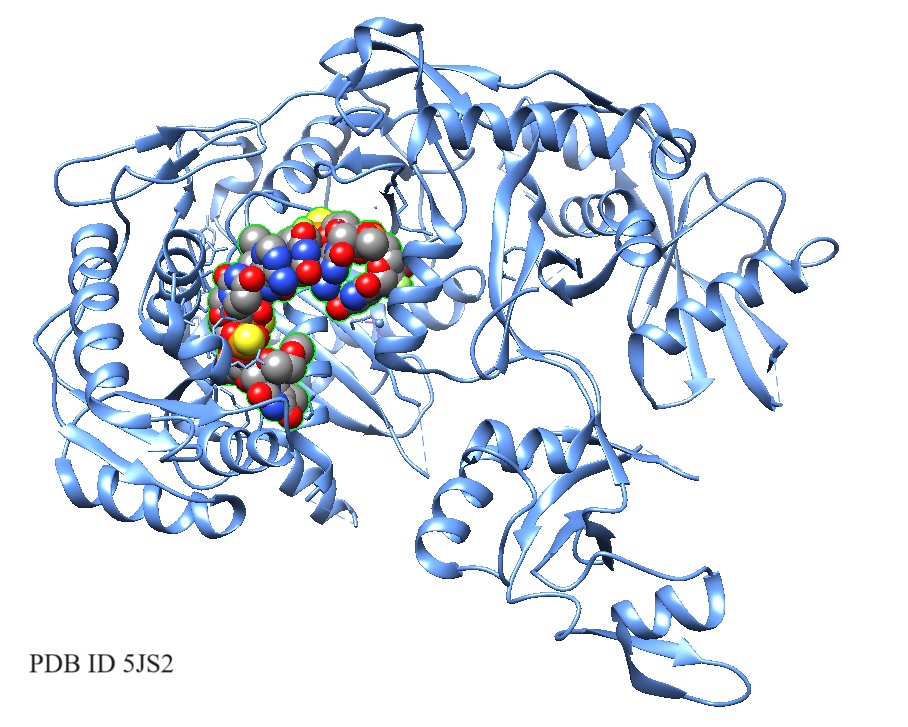
5JS2: Human Argonaute-2 Bound to a Modified siRNA.
|
Schirle NT, Kinberger GA, Murray HF, Lima WF, Prakash TP, MacRae IJ. Structural Analysis of Human Argonaute-2 Bound to a Modified siRNA Guide. J Am Chem Soc. 2016 Jul 20;138(28):8694-7. [PMC]
|
|
|
Schirle et al., in 2016, solved the crystal structure of human Ago2 bound to a metabolically stable siRNA containing extensive backbone modifications. Comparison to the structure of an equivalent unmodified-siRNA complex indicated that the structure of Ago2 is relatively unaffected by chemical modifications in the bound siRNA.
|
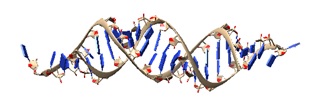
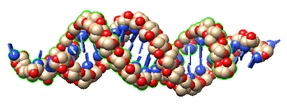
2F8S: Crystal structure of Aa-Ago with externally-bound siRNA.
|
Yuan YR, Pei Y, Chen HY, Tuschl T, Patel DJ. A potential protein-RNA recognition event along the RISC-loading pathway from the structure of A. aeolicus Argonaute with externally bound siRNA. Structure. 2006 Oct;14(10):1557-65. [PMC]
|
|
siRNA structural model from the crystal structures of Aa-Ago bound to 22-mer and 26-mer self-complementary siRNAs, identified as an externally bound siRNA-Ago complex.
The 2 nt 3′ overhang at one end of the siRNA inserts into a cavity positioned on the outer surface of the PAZ-containing lobe of the bilobal Aa-Ago architecture in both complexes.
|
Cell-penetrating peptide for cellular siRNA delivery
Cell-penetrating peptides (CPPs) are small peptides that trigger the movement of molecules across cell membranes. CPPs can penetrate the cell membrane and mitochondrial and nuclear membranes without destroying them. These short peptides can translocate and transport cargo molecules into the intracellular space by crossing biological membranes.
Known CPPs are all water soluble, relatively small, do not have more than 35 amino acid residues, and have a very low cytotoxicity. However, there exists no unique classification for these peptides. Categorization can be done according to their origin and ability to link with cargo molecules or structures.
Classification of CPPs by their structure divides CPPs into (1) cationic CPPs, (2) amphipathic CPPs, and (3) hydrophobic CPPs.
A list of CPPS is found here.
A ribbon model and a surface model of the cell-penetrating peptide RW16 is shown in the following figures.
6RQS: RW16 peptide
|
|
|
|
|
|
>pdb|6RQS|A Chain A, ARG-ARG-TRP-ARG-ARG-TRP-TRP-ARG-ARG-TRP-TRP-ARG-ARG-TRP-ARG-ARG
XXRRWRRWWRRWWRRWRR
|
Jobin ML, Vamparys L, Deniau R, Grélard A, Mackereth CD, Fuchs PFJ, Alves ID. Biophysical Insight on the Membrane Insertion of an Arginine-Rich Cell-Penetrating Peptide. Int J Mol Sci. 2019 Sep 9;20(18):4441. [PMC]
|
Mechanism of membrane insertion
Jobin et al. reported that side chain contacts, in both buried and solvent-exposed positions, contribute to the peptide's conformational stability and function. Three tryptophanes (Ws) form a hydrophobic pocket surrounding Arg15, masking the positive charges of the Arg (R) residue. The pairing of aromatic and polar residues decreased the energetic barrier for the motion of cationic side chains through a lower dielectric environment like the cell membrane bilayer. Similar π-cation interactions are also present in penetratin between R and multiple W residues.
According to Singh et al., conjugation of siRNA to most unmodified CPPs did not generate desirable effects due to either lack of serum stability, endosomal entrapment, or some other factor. However, chemical modifications of the CPPs and siRNAs enhanced the transfection efficiency.
Compared to other methods, CPP-mediated delivery methods may have advantages in terms of efficiency, siRNA-carrying capacity, and biocompatibility.
Unfortunately, our understanding of the CPP internalization pathway is complicated and needs to be fully understood.
Further research is needed to dissect the mechanism of CPP cellular uptake for designing CPPs with enhanced delivery and cell penetration capabilities. Overall, CPPs could provide new opportunities for systemic siRNA delivery.
Reference
Edinger, Daniel. "SiRNA Therapy for Cancer." 2013 Ph.D. Thesis.
Rathnayake PV, Gunathunge BG, Wimalasiri PN, Karunaratne DN, Ranatunga RJ. Trends in the Binding of Cell Penetrating Peptides to siRNA: A Molecular Docking Study. J Biophys. 2017; 2017:1059216. [PMC]
Singh T, Murthy ASN, Yang HJ, Im J. Versatility of cell-penetrating peptides for intracellular delivery of siRNA. Drug Deliv. 2018; 25(1):1996-2006. [PMC]
Takeuchi T, Futaki S. Current Understanding of Direct Translocation of Arginine-Rich Cell-Penetrating Peptides and Its Internalization Mechanisms. Chem Pharm Bull (Tokyo). 2016;64(10):1431-1437. [article].
---...---
Bio-Synthesis provides a full spectrum of bio-conjugation services including high quality custom oligonucleotide modification services, back-bone modifications, conjugation to fatty acids and lipids, cell-penetrating peptides, cholesterol, tocopherol, other peptides as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs, miRNA, or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---