RNA uridylation has now emerged as a widespread posttranscriptional regulator of gene expression. Single- and double-stranded RNAs available as custom RNAs allow the study of uridylation mechanisms. The availability of novel high throughput sequencing protocols revealed the pervasiveness of messenger RNA (mRNA) uridylation, ranging from plants to humans.
mRNAs in both prokaryotes and eukaryotes need to be resistant to decay to be translated but must eventually undergo degradation to allow appropriate regulation of gene expression.
A vital part of gene expression control is the degradation of messenger RNA (mRNA) involving the removal of a poly(A) tail in both prokaryotes and eukaryotes.
In 2008, Mullen and Marzluff discovered a new mechanism of mRNA decay. Histone mRNAs, which are never polyadenylated in mammalian cells, degrade by a cell cycle-regulated mechanism that involves addition of a short oligo (U) tail at the 3 end which is recognized by the Lsm1–7 complex, guiding the transcript into the standard mRNA decay pathways. The two researchers observed that the degradation of histone mRNAs requires the stem–loop sequence, which binds the stem–loop-binding protein (SLBP), active translation of the histone mRNA, and the location of the stem–loop close to the termination codon. The initial step in histone mRNA degradation is the addition of uridines to the 3′ end of the histone mRNA, both after inhibition of DNA replication and at the end of S phase.
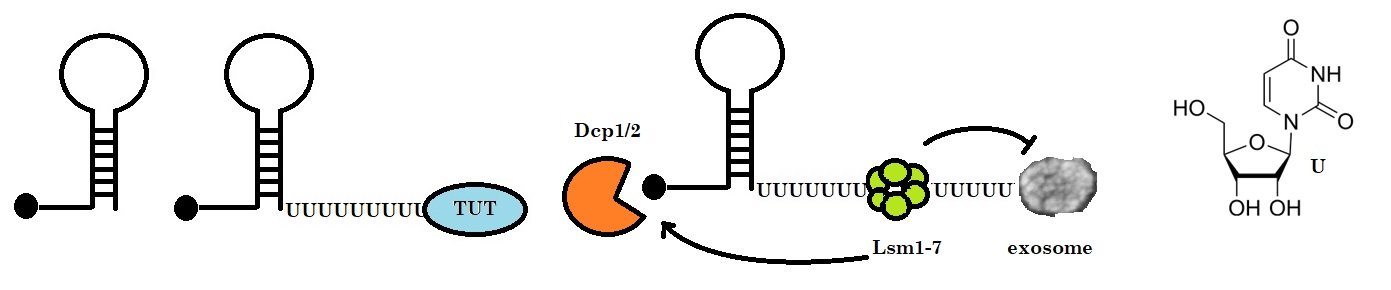
Figure 1: Histone mRNA decay. Histone mRNAs undergo oligouridylation by a cytoplasmic terminal uridyl transferase at the end of S phase leading to the association with Lsm1–7 and recruitment of the decapping and 5’–3’ decay machinery. Decay also occurs 3’–5’ by the exosome. How Lsm1–7 association influences exosome activity is unclear, although there is evidence for an inhibitory role (Adapted from Wilusz & Wilusz).
Uridylation refers to adding one or more uridine molecules to target molecules. RNA uridylation has been detected in many eukaryotes, including trypanosomes, animals, plants, and fungi, but not in budding yeast. All classes of eukaryotic RNAs can be uridylated.
Uridylation can tag viral RNAs. The untemplated addition of a few uridines at the 3' end of a transcript can determine the fate of these RNA. In rare instances, uridylation is an intrinsic step in the maturation of noncoding RNAs, such as the U6 spliceosomal RNA or mitochondrial guide RNAs in trypanosomes.
Uridylation can also switch specific miRNA precursors from a degradative to a processing mode. The switch depends on the number of uridines added, regulated by the cellular context. Typically, the uridylation of mature noncoding RNAs or their precursors accelerates their decay.
Uridylation also tags mRNAs.
High throughput sequencing protocols have recently revealed the pervasiveness of mRNA uridylation from plants to humans.
For noncoding RNAs, the primary function to date for mRNA uridylation is to promote degradation.
3' uridylation is an essential modification associated with coding and noncoding RNA degradation in eukaryotes.
Additional roles of U-tailing begin to emerge, such as the control of mRNA deadenylation, translation control, and possibly storage.
Scientists are just beginning to appreciate RNA uridylation's diverse roles and its full temporal and spatial implications in regulating gene expression.
In the adult human nervous system, uridine is the primary source of pyrimidine nucleosides. When taken up by the brain, uridine is phosphorylated to nucleotides. Phosphorylated uridine is a source for DNA and RNA synthesis, membrane parts, and glycosylation synthesis. Neural and glial cells can release uridine nucleotides and uridine diphosphate-sugars (UDP-sugars).
Recently Ye et al. suggested that uridine, when taken as a supplement, potentially prevents osteoarthritis (OA) induced by aging. The research group found that uridine can alleviate chondrocytes and mesenchymal stem cells (MSCs) in vivo. Their study indicated that uridine could relieve OA in vivo. These findings suggest that uridine, used as a functional food, could treat and prevent early aging and OA.
In 2012, a study by Heo et al. revealed the functional duality of uridylation. The research group identified the terminal uridylyl transferases TUT7/4/2 as parts of the miRNA biogenesis pathway. Terminal uridylyl transferases are responsible for pre-miRNA mono-uridylation. The TUTs act specifically on dsRNAs with a 1 nucleotide 3′-overhang, creating a 2 nt 3′-overhang. The depletion of TUTs reduces let-7 levels and disrupts let-7 function. Heo et al. noticed that group II pre-miRNAs acquire a shorter (1 nt) 3′ overhang during Drosha processing, requiring a 3′-end mono-uridylation for Dicer processing.
In 2014, Lee et al. reviewed the domain organization of five human terminal uridylyl transferases, also called “writers,” that uridylate RNAs. These writers of uridylation belong to a family of ribonucleotidyl transferases containing a catalytic domain with sequence homology to DNA polymerase β. Some ribonucleotidyl transferases have uridylation activity. Noncanonical poly(A) polymerases (PAPs) are also called terminal uridylyl transferases (TUTs) or poly(U) polymerases (PUPs). Humans have seven proteins with potential TUT activity potentially uridylating mRNAs or microRNAs.
In 2015, Kim et al. showed that terminal uridylyl transferases (TUTs) function as integral microRNA (miRNA) biogenesis regulators. The researchers utilized biochemistry, single-molecule, and deep sequencing techniques to investigate how human TUT7 (also known as ZCCHC6) recognizes and uridylates precursor miRNAs (pre-miRNAs) in the absence of Lin28. The study found that the overhang of a pre-miRNA is the key structural element recognized by TUT7 and its paralogues, TUT4 (ZCCHC11) and TUT2 (GLD2/PAPD4). This study revealed dual roles and mechanisms of uridylation in repairing and removing defective pre-miRNAs.
TUT7 catalyzed mono-uridylation restores the canonical end structure (2-nt 3′-overhang) of group II pre-miRNAs with a 1-nt 3′-overhang. This modification promotes miRNA biogenesis.
For pre-miRNAs where the 3′-end is further recessed into the stem (as in 3′-trimmed pre-miRNAs), TUT7 generates an oligo-U tail that leads to degradation.
TUT7 uses processive Lin28-stimulated oligo-uridylation for both mono- and oligo-uridylation in the absence of Lin28. The overhang length dictates the frequency (but not duration) of the TUT7-RNA interaction, explaining how TUT7 differentiates pre-miRNA species with different overhangs.
Also, in 2015, Song et al. showed that the posttranscriptional addition of nontemplated nucleotides to the 3′-ends of RNA molecules could significantly impact their stability and biological function. The researchers reported that the nontemplated addition of uridine or adenosine to the 3′-ends of RNAs occurs in different organisms ranging from algae to humans and on different kinds of RNAs, including histone mRNAs, mRNA fragments, U6 snRNA, mature small RNAs and their precursors as well as others.
These modifications lead to different outcomes, such as increasing RNA decay, promoting or inhibiting RNA processing, or changing RNA activity.
Modifications can be RNA sequence-specific and subjected to temporal or spatial regulation in development. RNA tailing and its outcomes have been associated with human diseases.
In 2016, Lee et al. reported that higher animals have multiple isoforms of let-7 miRNAs. The isoforms share a consensus sequence called the ‘seed sequence’ categorized as the let-7 miRNA family. The expression of the let-7 family is required for developmental timing and tumor suppressor function. However, for the self-renewal of stem cells, the let-7 expression must be suppressed. Because dysregulation of let-7 processing is deleterious, the biogenesis of let-7 is tightly regulated by cellular factors, such as by the RNA binding proteins LIN28A/B and DIS3L2.
In 2018, De Almeida et al. reviewed RNA uridylation as a potent and widespread posttranscriptional regulator of gene expression. The advent of novel high-throughput sequencing protocols has recently revealed the pervasiveness of mRNA uridylation in many organisms.
Reference
Bernstein, David L., Xinpei Jiang, and Slava Rom. 2021. "let-7 microRNAs: Their Role in Cerebral and Cardiovascular Diseases, Inflammation, Cancer, and Their Regulation" Biomedicines 9, no. 6: 606. [biomedicines, mdpi]
De Almeida C, Scheer H, Zuber H, Gagliardi D. RNA uridylation: a key posttranscriptional modification shaping the coding and noncoding transcriptome. Wiley Interdiscip Rev RNA. 2018 Jan;9(1). doi: 10.1002/wrna.1440. Epub 2017 Oct 5. PMID: 28984054. [pubmed]
Dobolyi A, Juhász G, Kovács Z, Kardos J. Uridine function in the central nervous system. Curr Top Med Chem. 2011;11(8):1058-67. doi: 10.2174/156802611795347618. PMID: 21401495. [pdf]
Glogovitis I, Yahubyan G, Würdinger T, Koppers-Lalic D, Baev V. isomiRs-Hidden Soldiers in the miRNA Regulatory Army, and How to Find Them? Biomolecules. 2020 Dec 30;11(1):41. doi: 10.3390/biom11010041. PMID: 33396892; PMCID: PMC7823672. [PMC]
Heo I, Ha M, Lim J, Yoon MJ, Park JE, Kwon SC, Chang H, Kim VN. Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II let-7 microRNAs. Cell. 2012 Oct 26;151(3):521-32. doi: 10.1016/j.cell.2012.09.022. Epub 2012 Oct 11. PMID: 23063654. [cell]
Kim B, Ha M, Loeff L, Chang H, Simanshu DK, Li S, Fareh M, Patel DJ, Joo C, Kim VN. TUT7 controls the fate of precursor microRNAs by using three different uridylation mechanisms. EMBO J. 2015 Jul 2;34(13):1801-15. [PMC]
Lee H, Han S, Kwon CS, Lee D. Biogenesis and regulation of the let-7 miRNAs and their functional implications. Protein Cell. 2016 Feb;7(2):100-13. [PMC]
Medhi R, Price J, Furlan G, Gorges B, Sapetschnig A, Miska EA. RNA uridyl transferases TUT4/7 differentially regulate miRNA variants depending on the cancer cell type. RNA. 2022 Mar;28(3):353-370. [PMC]
Menezes MR, Balzeau J, Hagan JP. 3' RNA Uridylation in Epitranscriptomics, Gene Regulation, and Disease. Front Mol Biosci. 2018 Jul 13;5:61. [PMC]
Mihye Lee, Boseon Kim, V. Narry Kim; Emerging Roles of RNA Modification: m6A and U-Tail. Cell, Volume 158, Issue 5, 2014, Pages 980-987. [cell,sciencedirect]
Mullen, T.E. and Marzluff, W.F. 2008. Degradation of histone mRNA requires oligouridylation followed by decapping and simultaneous degradation of the mRNA both 5 to 3 and 3 to 5. Genes & Dev. 2008, 22(1): 50-65.
Qian Hu, Huiru Yang, Mingwei Li, Lingru Zhu, Mengqi Lv, Fudong Li, Zhiyong Zhang, Guodong Ren, Qingguo Gong, Molecular mechanism underlying the di-uridylation activity of Arabidopsis TUTase URT1, Nucleic Acids Research, Volume 50, Issue 18, 14 October 2022, Pages 10614–10625. [NAR]
Song J, Song J, Mo B, Chen X. Uridylation and adenylation of RNAs. Sci China Life Sci. 2015 Nov;58(11):1057-66. [PMC]
Wang, Y., Weng, C., Chen, X. et al. CDE-1 suppresses the production of risiRNA by coupling polyuridylation and degradation of rRNA. BMC Biol 18, 115 (2020). [BMC,biomedcentral]
Warkocki Z, Krawczyk PS, Adamska D, Bijata K, Garcia-Perez JL, Dziembowski A. Uridylation by TUT4/7 Restricts Retrotransposition of Human LINE-1s. Cell. 2018 Sep 6;174(6):1537-1548. [PMC]
Wilusz, C.J., and Wilusz J.; New ways to meet your (3’) end—oligouridylation as a step on the path to destruction. GENES & DEVELOPMENT 22:1–7, 2008. [PMC]
Yamashita, S., Nagaike, T. & Tomita, K. Crystal structure of the Lin28-interacting module of human terminal uridylyltransferase that regulates let-7 expression. Nat Commun 10, 1960 (2019). [nature]
Ye J, Jin Z, Chen S, Guo W. Uridine relieves MSCs and chondrocyte senescence in vitvo and exhibits the potential to treat osteoarthritis in vivo. Cell Cycle. 2022 Jan;21(1):33-48. [PMC]
Zhu L, Hu Q, Cheng L, Jiang Y, Lv M, Liu Y, Li F, Shi Y, Gong Q. Crystal structure of Arabidopsis terminal uridylyl transferase URT1. Biochem Biophys Res Commun. 2020 Apr 2;524(2):490-496. [PubMed]
Zigáčková D, Vaňáčová Š. The role of 3' end uridylation in RNA metabolism and cellular physiology. Philos Trans R Soc Lond B Biol Sci. 2018 Nov 5;373(1762):20180171. [PMC]
---...---