The efficient delivery of RNA molecules into cells or tissue, such as siRNAs, is a necessity for the development of therapeutic RNAs. For many years, the difficulty encountered in efficient and safe in vivo delivery of RNAs prevented the widespread clinical use of siRNA-based therapies until recently.
The first RNA interference (RNAi) drug Onpattro for the treatment of polyneuropathies was approved by the FDA in 2018.
Already, siRNAs for the SARS coronavirus targeting the regions in the genome encoding spike protein and ORF1b (NSP12) have been designed as well, one targeting the ‘Leader’ sequence that could potentially suppress the replication of SARS coronaviruses.
To improve cellular uptake of small interfering RNAs (siRNAs), Osborn et al. studied the effect of lipid conjugated siRNAs on cellular endogenous lipid transport pathways to determine the biodistribution of conjugated siRNAs.
For the design and synthesis of lipid hydrophobically modified RNA conjugates (hsiRNAs), the researchers used a panel of biologically occurring, but structurally diverse lipids. A clinically validated, chemically altered siRNA scaffold is at the heart of the design. Earlier studies established that cholesterol-conjugated siRNAs have an 8- to 15-fold higher activity when pre-complexed into purified high-density lipoprotein (HDL) than when injected alone. Recent studies indicated that the asialoglycoprotein receptor (ASGPR) contributes to the uptake of unconjugated phosphorothioate-modified antisense oligonucleotides (PS ASOs). The most clinically advanced trivalent N-acetylgalactosamine (GalNac)-siRNA binds to ASGPR on hepatocytes with high selectivity triggering a potent and durable gene silencing in patients. Lipids, when conjugated to siRNA molecules, enhance circulation time, and promote local and systemic delivery and efficacy. For example, cholesterol-modified siRNA silence liver apolipoprotein B (ApoB) expression at high doses.
Lipids, Linkers and Modification for the design of hsiRNA conjugates
| Lipids used: | Lithocholic acid (LCA), Docosahexaenoic acid (DHA), Docosanoic acid (DCA), Cholesterol (Chol). |
| Linker used: | Triethylene glycol (TEG). |
| Modifications used: | 2’-Fluoro RNA, 2’-O-Methyl RNA, Cholesterol, Phosphorothioate, TEG linker, 5’-Vinylphosphonate. |
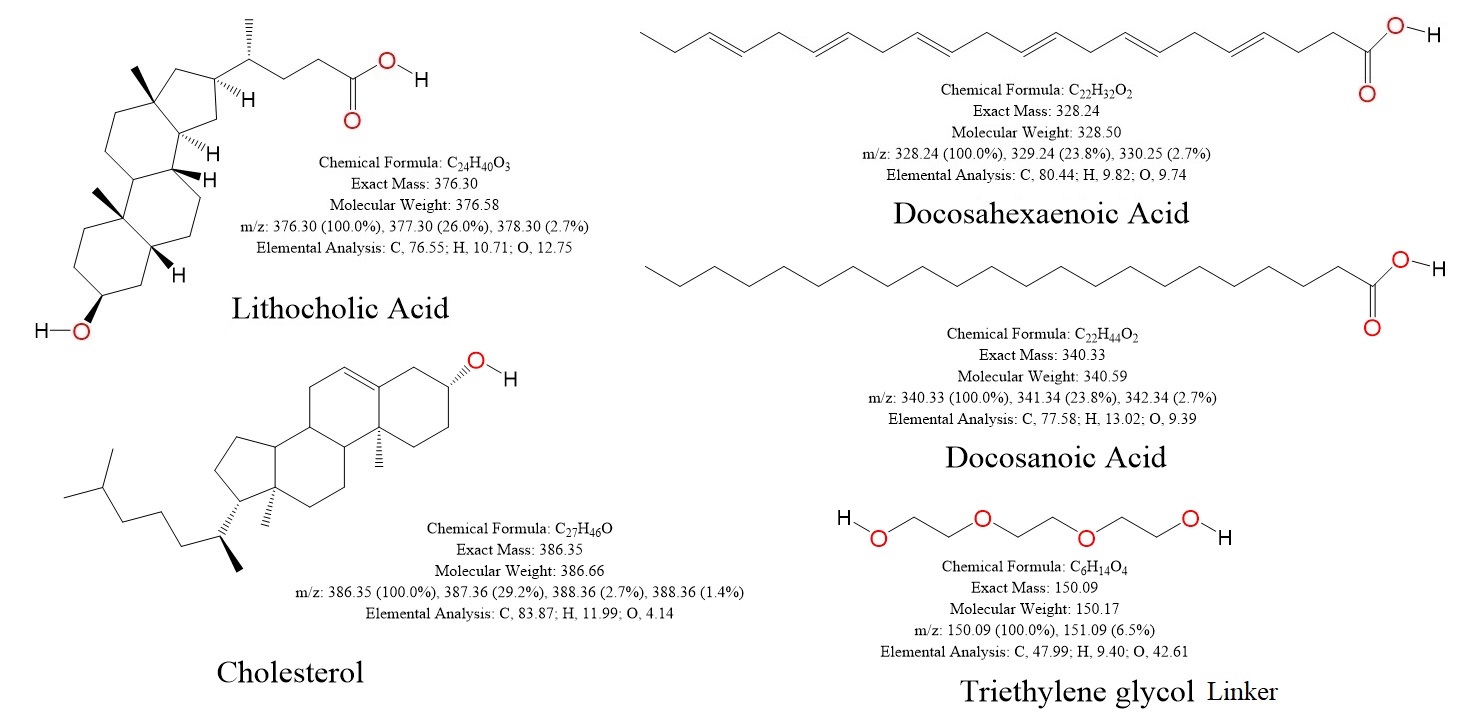 Figure 1: Chemical structures of lipids and linker used for conjugation of siRNAs.
Figure 1: Chemical structures of lipids and linker used for conjugation of siRNAs.
Design of Lipid-hsiRNA conjugates
The scaffold used for the design of lipid-hsiRNA conjugates consists of a hydrophobically modified siRNA scaffold, called hsiRNA. The conjugate contains a 20-nucleotide (nt) antisense or guide strand and a 15-nt sense or passenger strand with alternating 2’-O-methyl and 2’-fluoro sugar modifications. The 5’-end of the antisense strand contains the stable phosphate mimic (E)-vinylphosphonate (VP). The addition of VP to the 5’-end of the guide strand increases siRNA tissue accumulation, extends silencing activity duration, and shields oligonucleotides from exonuclease-mediated degradation, thereby promoting cellular internalization. Because of the rapid degradation and clearance of unmodified oligonucleotides, these modifications stabilize siRNAs and prevent their degradation in cells and tissue. The 3’-end of the sense strand is an optimal position for the attachment of conjugates since it has a minimal effect on siRNA-RISC or intracellular RNA-induced silencing complex loading. The use of linkers, such as the TEG linker, allows attachment of lipid moieties.
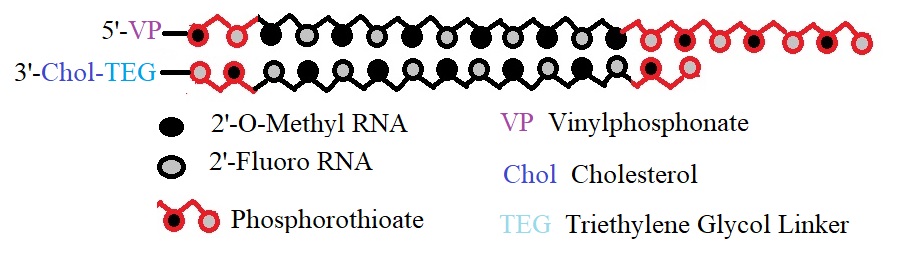
Figure 2: Modification pattern of lipid-hsiRNA.
Osborn et al. reported that lipid-conjugated hsiRNA engage distinct lipid transport pathways and elicit silencing in a variety of tissues with minimal systemic toxicity.
Reference
Feingold, K.R., & Grunfeld, C.; Introduction to Lipids and Lipoproteins. NIH ENDOTEXT.
Osborn MF, Coles AH, Biscans A, Haraszti RA, Roux L, Davis S, Ly S, Echeverria D, Hassler MR, Godinho BMDC, Nikan M, Khvorova A. Hydrophobicity drives the systemic distribution of lipid-conjugated siRNAs via lipid transport pathways. Nucleic Acids Res. 2019 Feb 20;47(3):1070-1081. [PMC]
Bio-Synthesis Inc. is pleased to offer a large variety of oligonucleotides and peptides for a number of research applications, including COVID 19 testing, analysis and vaccine development!
---...---