Rare-earth elements, such as permanent magnets, light-emitting diodes, and phosphors, are widely used in modern technologies. However, effective separation of lanthanides is challenging. The increased demand for rare-earth elements led to environmental exposure and water pollution from rare-earth metal mines and various commercial products. The development of safe technology for separating and adsorbing rare-earth elements promises a green chemistry approach, potentially allowing the removal of environmental pollution.
Rare-earth metals include the elements scandium, yttrium, lanthanum, cerium, praseodymium, neodymium, promethium, samarium, europium, gadolinium, terbium, dysprosium, holmium, erbium, thulium, ytterbium, lutetium. The elements lanthanum to lutetium in the periodic system are known as lanthanoids.

Analytically, rare-earth elements are virtually inseparable because of their similar chemical properties. However, their electronic and magnetic properties allow for unique technological applications.
The separation of these lanthanoids is complicated because of the similar physicochemical properties of their predominating three plus (3+, or III) ions. The ionic radii decrease only 0.19 Å between LaIII and LuIII, leading to these metals co-occurring in rare-earth-bearing minerals.
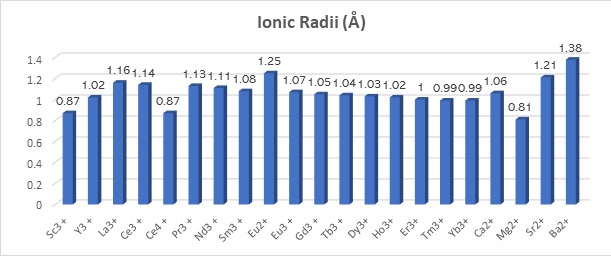
Data source for ionic radii (Shannon, R.D., 1976; Mattocks et al. 2023.).
Recently, in 2018, Cotruvo et al. reported the identification of a new highly selective lanthanum (LnIII)-binding protein in the model methylotroph, Methylobacterium extorquens, which the researchers named lanmodulin. Lanmodulin is a high-affinity lanthanide-binding protein identified in the bacterium Methylobacterioum extorquens. This lanthanide-modulated protein contains metal coordination motifs known as EF-hands. EF-hands are associated with nanomolar- to millimolar-affinity towards calcium ions. However, lanmodulin responds to lanthanum ions with a 108-fold selectivity over calcium ions. Lanthanum (La) has an atomic number of 57; its usual oxidation state is three plus (3+). A biological role for La in humans is not known but is essential in some bacteria.
The research group purified the protein from M. extorquens AM1 cells grown in 1 μM LaCl3 and methanol. Classical ammonium sulfate precipitation followed by cation exchange chromatography (CIX) and size exclusion chromatography (SEC) resulted in a highly purified protein. After sequence analysis, the scientists expressed the protein in E. coli for biochemical characterization studies.
Mattocks et al., in 2019, created a highly selective, genetically encoded fluorescent sensor for lanthanoids called LaMP1. The researchers created LaMP1 by replacing the CaM and M13 domains of the FRET-based biosensor Cameleon D2 (ECFP and Citrine, λex = 433 nm; λem = 475 nm, λem = 529 nm) with lanmodulin (A22–R133).
LaMP1 showed FRET ratio changes (Rf/R0) from ~5.4 to 6.7 with picomolar affinity (Kd = 9.4–44 pM) for all the rare earth elements and selectivity over Ca2+ (Rf/R0 ~ 2.9; Kd = 1.2 mM) at pH 7.2. Little to no interference was observed from environmentally and biologically relevant cations, including Fe3+, Al3+, Mn2+, Cu2+, Mg2+, Na+, and K+. As a result, LaMP1 can be used as a probe for extracellular and intracellular Ln3+ ion homeostasis in live M. extorquens using a fluorescence plate reader assay.
Also in 2019, Cook et al. reported the NMR solution structure of lanmodulin complexed with yttrium III [PDB ID 6MI5]. The structure revealed an unusual fusion of adjacent EF-hand motifs, resulting in a compact fold of EF-hand motifs. Also, an additional carboxylate ligand contributes to the protein's picomolar affinity for lanthanide ions, suggesting a role of unusual N i+1-H···N i hydrogen bonds, in which lanmodulin's EF-hand proline residues participate in selective lanthanide ion recognition.
The EF-hand motif is a common calcium-binding motif found in many proteins. The lanmodulin sequence contains four predicted EF-hand motifs: EF1, EF2, EF3, and EF4. The protein binds three equivalents of lanthanum 3+ ions with picomolar affinity and a fourth with approximately micromolar affinity.
The research group cloned the lanmodulin protein using the predicted sequence [residues 1−21 are a signal peptide].
Protein sequence:
>WP_253389665.1 EF-hand domain-containing protein [Methylorubrum extorquens]
MAFRLSSAVLLAALVAAPAYAAPSTTTKVDIAAFDPDKDGTIDLKEALAAGSAAFDKLDPDKDGTLDAKE
Signal peptide EF1 EF2
LKGRVSEADLKKLDPDNDGTLDKKEYLAAVEAQFKAANPDNDGTIDARELASPAGSALVNLIR
EF3 EF4
Cloned protein:
>pdb|6MI5|X Chain X, Lanmodulin
PTTTTKVDIAAFDPDKDGTIDLKEALAAGSAAFDKLDPDKDGTLDAKELKGRVSEADLKKLDPDNDGTLD KKEYLAAVEAQFKAANPDNDGTIDARELASPAGSALVNLIRHHHHHH
Cook et al. generated a construct for the expression of the protein in Escherichia coli comprising an N-terminal methionine residue, residues 22−133, and a C-terminal hexa-histidine tag. However, according to mass spectrometric analysis, the first two residues (Met and Ala22) are cleaved during expression. The construct exhibited metal-dependent secondary structural changes and Ln3+ affinities comparable to untagged, wild-type lanmodulin.
In 2023, Mattocks et al. reported X-ray structures of lanmodulin constructs in complex with neodymium 3+ ions [PDB ID 8FNS], dysprosium 3+ ions at pH 7 [PDB ID 8FNR], and lanthanum 3+ ions at pH 7 [PDB ID 8DQ2].
Reference
Cook EC, Featherston ER, Showalter SA, Cotruvo JA Jr. Structural Basis for Rare Earth Element Recognition by Methylobacterium extorquens Lanmodulin. Biochemistry. 2019 Jan 15;58(2):120-125. [ACS] [PDB ID: 6MI5].
Cotruvo, J. A., Jr., Featherston, E. R., Mattocks, J. A., Ho, J. V., and Laremore, T. N. (2018) Lanmodulin: A highly selective lanthanide-binding protein from a lanthanide-utilizing bacterium. J. Am. Chem. Soc., 2018, 140, 44, 15056-15061 [ACS].
Lewit-Bentley A, Réty S. EF-hand calcium-binding proteins. Curr Opin Struct Biol. 2000 Dec;10(6):637-43. [Sciencedirect].
Mattocks, J.A., Ho, J.V. and Cotruvo, J.A.Jr. (2019) J. Am. Chem. Soc., 141, 2857–2861. [PubMed]
Mattocks JA, Jung JJ, Lin CY, Dong Z, Yennawar NH, Featherston ER, Kang-Yun CS, Hamilton TA, Park DM, Boal AK, Cotruvo JA Jr. Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer. Nature. 2023 Jun;618(7963):87-93. [PMC]
Shannon, R.D., 1976. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A32, 751–767. [AC]
Snyder EE, Buoscio BW, Falke JJ. Calcium(II) site specificity: effect of size and charge on metal ion binding to an EF-hand-like site. Biochemistry. 1990 Apr 24;29(16):3937-43. [PMC]
---...---