Fluorescent probes allow detection of protein location and activation, help identify protein complex formation and conformational changes as well as monitoring biological processes in vivo. Fluorescent probes are biosensor molecules. Biosensor molecules allow direct detection of analytes or target molecules.
A molecular biosensor contains a biological recognition element called a receptor covalently connected to a transducer molecule. The transducer is generally a fluorophore needed for signaling. The receptor molecule is selected or designed, such as to recognize the target molecule or analyte specifically. Upon binding to the target, the fluorophore responds to the local environment transforming the recognition event into a measurable signal. Fluorescent molecular biosensors allow detection and quantification of analytes. In general, target molecules are nucleic acids, DNA or RNA, or proteins, often enzymes or antibodies. Monitoring the peptide loading process onto class II MHC proteins is an example for the design of fluorogenic biosensor molecules.
Monitoring the peptide loading process onto class II MHC proteins
To visualize the peptide loading process onto class II MHC proteins, Venkatraman et al. designed a series of novel fluorogenic probes that incorporate the environment-sensitive amino acid analogs 6-N,N-dimethylamino-2-3-naphthalimidoalanine and 4-N,N-dimethylaminophthalimidoalanine. When these fluorophores bind to the protein, they experience substantial changes in emission spectra, quantum yield, and fluorescence lifetime.
Peptides containing these fluorophores bind specifically to class II MHC proteins on antigen-presenting cells allowing the monitoring of peptide binding in vivo. Venkatraman et al. used these probes to track developmentally regulated cell-surface peptide-binding activity in primary human monocyte-derived dendritic cells.
VKQNTLKLAT.jpg)
Figure 1: Structure of the Ac-PK(4-DAPA)VKQNTLKLAT peptide.
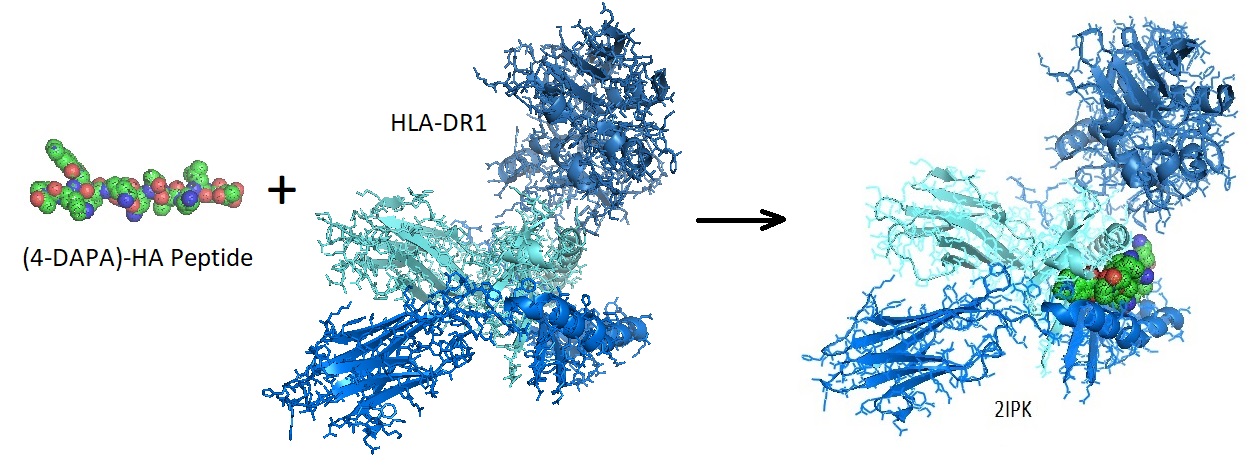
Figure 2: Binding of a fluorophore-peptide to HLA-DR1. The crystal structure of the Mhc Class II Molecule Hla-dr1 in complex with the fluorogenic 14 mer peptide, AcPKXVKQNTLKLAT (x=3-[5-(dimethylamino)-1,3- Dioxo-1,3-dihydro-2h-isoindol-2-yl]-l-alanine) And The Superantigen, Sec3 Variant 3b2 [2IPK].
(4-DAPA)-fluorophore peptides bind specifically to class II MHC proteins on antigen-presenting cells. The fluorescent peptides allow following peptide binding in vivo as well as tracking developmentally regulated cell-surface peptide-binding activity in primary human monocyte-derived dendritic cells. Peptides designed similarly enable the development of a myriad of fluorogenic peptide probes utilizing a wide range of peptide-based recognition events. Examples are peptide binding to antibodies, other binding proteins, protease-specific peptide sequences, and epitope-derived peptide sequences.
Reference
Venkatraman P, Nguyen TT, Sainlos M, Bilsel O, Chitta S, Imperiali B, Stern LJ. Fluorogenic probes for monitoring peptide binding to class II MHC proteins in living cells. Nat Chem Biol. 2007 Apr;3(4):222-8. doi: 10.1038/nchembio868. Epub 2007 Mar 11. PMID: 17351628; PMCID: PMC3444530. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3444530/?report=reader
---…---