Oligonucleotide-conjugated antibodies are valuable tools for protein diagnostics and therapeutics. Oligonucleotide-conjugated antibodies increase the sensitivity of protein assays tremendously and allow multiplexing approaches. Oligonucleotide-conjugated antibodies also allow therapeutic cell targeting with therapeutic antibody-drug conjugates (ADCs). Other applications for oligonucleotide-conjugated antibodies are immune-PCR, hybridization chain reactions technics, enzyme activity studies, sequential fluorescence hybridization methods, and applications combining the conjugates with next-generation sequencing (NGS).
Since site-specific conjugation strategies based on protein tags or unnatural amino acids require recombinant cloning steps, non-site-specific conjugation methods allow the construction of large libraries of oligonucleotide-conjugated antibodies.
Recently, Wiener et al. combined a non-site-directed antibody conjugation method using copper-free click chemistry with ion-exchange chromatography to obtain purified single and double oligonucleotide-conjugated antibodies.
The research group utilized the copper-free click chemistry reaction to obtain single, double, and multiple oligonucleotide-conjugated antibodies. The cross-linking of dibenzocyclooctyne (DBCO) molecules to the antibodies' amino side chains using a hydroxysuccinimide (NHS) reaction allowed functionalization of the antibodies. Employing 3-azidopropionic acid sulfo-NHS ester (3AA-NHS) allowed the functionalization of amine-modified oligonucleotides. In this approach, first, the antibodies are reacted with DBCO-NHS ester. Mixing azide-modified oligonucleotides to DBCO-antibodies generated the conjugates. Oligonucleotides used in this study carried 5’-amino modifier C6 dT and were double HPLC-purified.
DBCO Copper-Free Click Reaction
Step 1: Activate and functionalize the antibody with DBCO.
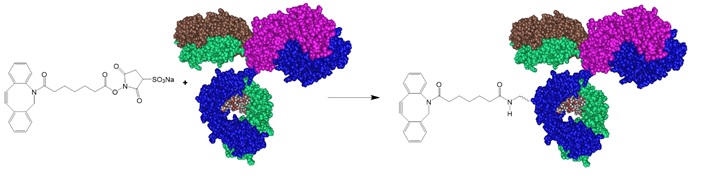
The research group recommended removing unreacted reagents via dialysis, spin-column-based gel-filtration desalting chromatography, spin column-based ultrafiltration, and cut-off membranes with a 3 or 7 kDA molecular weight cut-off.
Step 2: Activate and functionalize the oligonucleotide with an azide group.
.jpg)
The research group recommended removing unreacted reagents via dialysis, spin-column-based gel-filtration desalting chromatography, spin column-based ultrafiltration, and cut-off membranes with a 3 or 7 kDA molecular weight cut-off.
Step 3: Mix the two activated biomolecules to form the conjugate.
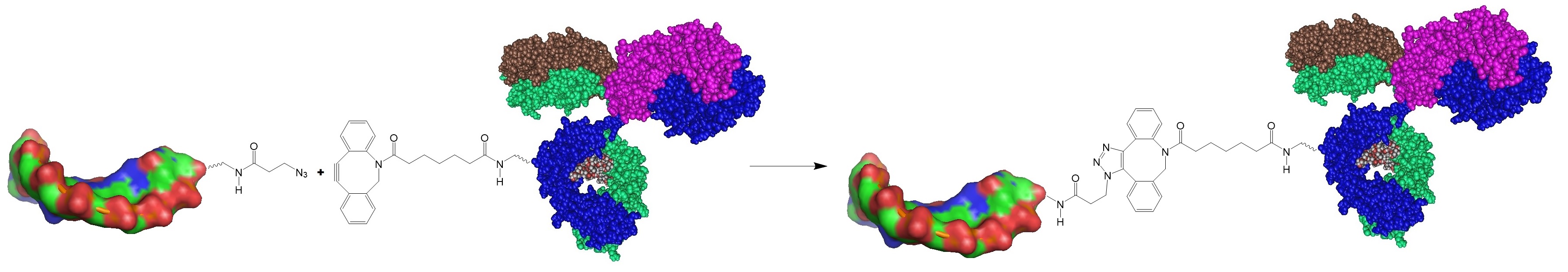
Step 4: Remove excess of azide or DBCO activated biomolecule with a scavenger molecule, via dialysis or purification. Analyze the resulting conjugates via ion exchange chromatography, SDS-electrophoresis or matrix assisted time-of-flight mass spectrometry (MALDI-TOF-MS).
A dialysis step allowed the removal of excess reagents. Ion exchange chromatography enabled the separation of antibody-oligonucleotides conjugates and guided the selection of reaction conditions to improve yields.
The researchers reported that this conjugation workflow allows the conjugation of multiple antibodies in various quantities with quantities as low as 25 ng and a yield of 5 ng.
For quantitative protein detection via amplification with antibody-oligonucleotide conjugates, the purity and the number of conjugated oligonucleotides need to be precisely controlled.
Reference
Wiener J, Kokotek D, Rosowski S, Lickert H, Meier M. Preparation of single- and double-oligonucleotide antibody conjugates and their application for protein analytics. Sci Rep. 2020 Jan 29;10(1):1457. [PMC]
---...---
Bio-Synthesis provides a full spectrum of bio-conjugation services including high quality custom oligonucleotide modification services, back-bone modifications, conjugation to antibodies, fatty acids and lipids, cholesterol, tocopherol, peptides, other proteins, as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---