Peptide-conjugated antisense oligonucleotides enable exon-skipping therapeutics.
Antisense Oligonucleotides (ASOs or AONs) are small single stranded chemically modified nucleic acids designed for targeting specific gene transcripts. The small size of the ASO is crucial for cell delivery.
Chemical modification of the ASO affects stability, solubility, toxicity, affinity, and resistance to degradation. The conjugation of peptides to oligonucleotides allows the generation of peptide-conjugated antisense oligonucleotides. Modifying the backbone of ASOs increases their stability and affinity to target sequences. Peptide-conjugated antisense oligonucleotides potentially enable the systemic correction of the Duchenne muscular dystrophy phenotype. Also, the conjugation of ASOs to arginine-rich cell-penetrating peptides improves their cell transduction properties.
ASOs targeting exon 53 of dystrophin pre-mRNA on the Duchenne muscular dystrophy (DMD) gene alter pre-mRNA splicing by removing specific exons in the dystrophin mRNA during the splicing process. The ASO hides the exon splicing sites from the splicing machinery and restores the reading frame of the transcript. Examples are the FDA-approved phosphorodiamidate morpholino ASOs viltolarsen and golodirsen.
Duchenne muscular dystrophy is a severe muscle degenerative X-linked allelic disorder resulting in the absence of functional dystrophin. The human gene called dystrophin (DMD) spans 24 kb of genomic DNA with its 79 exons. It encodes dystrophin, a 427 kDa protein localized on the cytoplasmic side of the sarcolemma of skeletal and cardiac muscle fibers and the cortical/cerebellar synapses.
Utilizing polyclonal antibodies directed against fusion proteins that contained two distinct regions of the mDMD cDNA, Hoffman et al., in 1987, identified the protein product of human Duchenne muscular locus (DMD) and its mouse homolog (mDMD). A mutation disrupting the reading frame in the dystrophin gene results in the absence of the functioning protein. Non-randomly distributed deletions involving one or more exons are the most common mutations.
Exon skipping is a novel therapeutic approach to correct mutations in DMD patients and restore the proper expression of dystrophin. During the last decade, researchers investigated exon skipping ASOs as tools for the correction of missplicing diseases. ASOs can correct splicing defects through exon skipping.
ASOs targeting exon 53 of dystrophin pre-mRNA on the Duchenne muscular dystrophy (DMD) gene alter pre-mRNA splicing by removing specific exons in the dystrophin mRNA during the splicing process. The ASOS hides the exon spicing sites from the splicing machinery and restores the reading frame of the transcript. Examples are the FDA-approved phosphorodiamidate morpholino ASOs viltolarsen and golodirsen.
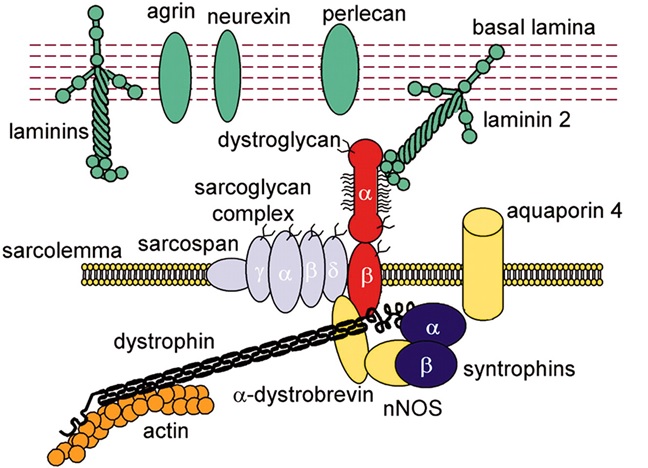
Figure 1: Extracellular membrane and cytoplasmic components of the dystrophin-glycoprotein complex (DGC), cardiac and skeletal muscle membrane specialization (Wiki Commons).
The DGC is a multimolecular complex linking the extracellular matrix to the cytoskeleton. The DGC is essential for the maintenance of normal cardiac and skeletal muscle. The DGC is a mechanosignaling complex that has mechanical and nonmechanical membrane-stabilizing functions. Destabilization of the DGC leads to membrane fragility and loss of membrane integrity. The result is a degeneration of skeletal muscle and cardiomyocytes.The cytoskeletal protein dystrophin links cytoplasmic γ-actin to the transmembrane components of the DGC. Dystrophin also binds to β-dystroglycan, and dystroglycan binds to the extracellular matrix protein laminin-α2. The sarcoglycan complex is composed of multiple subunits.
Yin et al., in 2008, reported the systemic correction of dystrophin expression in the muscle cells of adult dystrophic mdx mice. A single dose of a peptide-conjugated morpholino phosphoroamidate antisense oligonucleotide was sufficient to restore dystrophin protein expression. The treatment resulted in the improvement of muscle function in the mice. Since this work, antisense oligonucleotides have become the basis of exon skipping therapeutic approaches to correct defects in splicing events and repair the disrupted open reading frame.
However, the therapeutical use of unmodified ASOs is quite limited because they are subject to degradation by endonucleases and exonucleases. Therefore, in recent years researcher scientists have tested multiple chemical modifications of the phosphoribose backbone to improve the stability, efficacy, and pharmacokinetics of ASOs. To allow for high-affinity interaction with target mRNA during splicing, ASOs should not support ribonuclease H activity. Several studies tested ASOs containing 2’-O-methyl (2’-OMe) and 2’-O-methoxyethyl (2’-MOE) with a phosphorothioate backbone (PS) in cells. Further investigations revealed that ASOs containing bridged nucleic acids (BNAs, LNAs), peptide nucleic acids (PNAs), and phosphorodiamidate morpholinos (Mos) are also potent ASOs in cells. The non-ionic nature of the phosphorodiamidate linkage minimizes nuclear uptake. Adding charged cell-penetrating peptides to ASOs improves their uptake by cells.
The conjugation of therapeutic ASOs to delivery systems for enhanced uptake by skeletal and heart muscles is presently an active field. Conjugation of cell-penetrating peptides as carrier molecules to ASOs promises enhanced uptake of the ASOs in muscle tissues and cells. Yin et al. demonstrated that antisense oligonucleotides enable the exclusion of specific dystrophin exons to restore the open reading frame of dystrophin. The research group designed a peptide-ASO (PMO) conjugate by conjugating arginine-rich peptide mimics to a particular antisense oligonucleotide. The selected ASO targeted the murine dystrophin exon 23 5’-splice donor site. The systemic intravenous delivery into adult mdx mice allowed evaluation of how the ASO affected gene restoration. Mdx mice treated with the P007-PMO ASO showed near-normal levels of dystrophin expression in muscle tissue. Also, the treatment did not induce any noticeable toxic effects.
The P007 peptide conjugate increased the cell uptake of the ASO and restored dystrophin expression. However, more research is needed to show that ASO-based therapeutics are effective and safe in humans. Already several ASO-based drugs are in clinical trials.
Table 1: Oligonucleotide and peptides used for the ASO
|
Molecule
|
Sequence
|
Length
|
Name
|
|
|
|
|
|
|
ASO (PMO)
|
5′-GGCCAAACCTCGGCTTACCTGAAAT-3′
|
25
|
M23D
|
|
(RXR)4XB
|
N-RXRRXRRXRRXRXB-C
|
14
|
P007
|
|
(RXRRBR)2XB
|
N-RXRRBRRXRRBRXB-C
|
14
|
B peptide
|
R, L-arginine; X, 6-aminohexanoic acid (6AHA); B, β-alanine.
Reference
Karen A. Lapidos, Rahul Kakkar, and Elizabeth M. McNally; The Dystrophin Glycoprotein Complex. Signaling Strength and Integrity for the Sarcolemma. 94:1023–1031. [Circulating Research]
Myotonic Dystrophy Drug Development Pipeline 2021 [pdf]
HaiFang Yin, Hong M. Moulton, Yiqi Seow, Corinne Boyd, Jordan Boutilier, Patrick Iverson, Matthew J.A. Wood, Cell-penetrating peptide-conjugated antisense oligonucleotides restore systemic muscle and cardiac dystrophin expression and function, Human Molecular Genetics, Volume 17, Issue 24, 15 December 2008, Pages 3909–3918, [Oxford Academic]
DMD dystrophin [gene]
Golodirsen [drugbank]
Viltolarsen [drugbank]
---...---