The search is on for better options in antisense technology because significant obstacles still prevent the specific delivery of antisense oligonucleotides (ASOs) into many selected tissues or cells. For the development of cost-efficient oligonucleotide-based therapeutics, the following points still need to be addressed:
- Large-scale production,
- the toxicity of ASOs,
- the localization of oligonucleotides in specific cellular compartments or tissues, and
- the high cost of treatment.
However, a new type of ASOs called Thiophosphoramidate Morpholino Oligonucleotides could solve many of these problems. The chemical structures for a PMO, a PM, and a TMO are illustrated in figure 1.
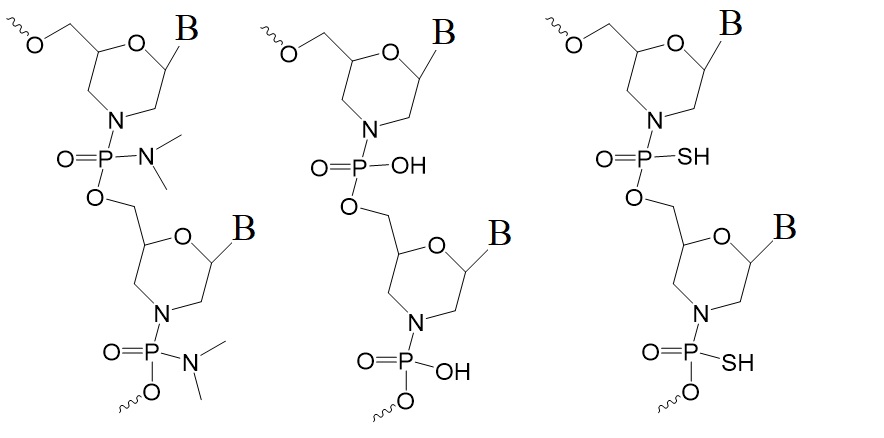
Figure 1: Chemical structures of phosphorodiamidate morpholino (left, PMO), phosphoramidate morpholino (middle, PM), and thiophosphoramidate morpholino (right, TMO).
Antisense morpholino oligomers (MOs) are molecular tools that allow selective gene knockdown. Morpholinos are synthetic antisense oligonucleotides, approximately 25 nucleotides in length. MOs have allowed antisense knockdown studies and screens in zebrafish (Danio rerio, Bill et al. 2009). The major antisense compounds utilized in zebrafish studies are morpholino phosphorodiamidate oligonucleotides. These molecules comprise a phosphorodiamidate backbone with a morpholine ring DNA bases. MOs function through the steric hindrance of the normal endogenous splicing or translation mechanisms. MOs target the splice donor site by inhibiting the binding of the U1 complex by inhibiting lariat formation and incorporation of the intron. Correctly designed MOs bind and block the translation initiation complex of messenger RNA (mRNA) sequences. ATG MOs block the initiation of protein translation. The result is a knockdown of a targeted protein. Splice-blocking MOs interfere with RNA splicing, resulting in truncated proteins, and target protector MOs block specific endogenous microRNAs (miRNA), stabilizing particular mRNA transcripts.
It appears that MOs do have a few side effects. For example, Bile et al. reported that not all MOs cause nonspecific events; however, some MOs correlate with aberrant morphologies with increased cell death, indicating off-targeting effects. Also, there is evidence that the impact of MOs only lasts up to five days, making them unsuitable for studying gene function at later stages of development. Further, MO effects do not always correspond to the observed phenotype of true mutants. Gentsch et al., in 2018, reported another example of a side effect. Embryos injected with control or target MOs to knockdown mesoderm-specific Brachyury paralogs showed a systemic GC content-dependent immune response and many off-target splicing defects. Homologs of the T-box gene Brachyury play essential roles in mesoderm differentiation and other aspects of early development in all bilaterians. Bilateral organisms contain a single plane of symmetry, body shapes that are mirror images along a midline called the sagittal plane. Examples are flatworms, clams, snails, octopuses, and others. Also, Gerety & Wilkinson, in 2011, reported that many MOs could have off-target effects mediated by the activation of tumor protein p53 (Tp53) and inducing apoptosis.
To address these issues, Langner et al. set out to develop a new type of ASOs called "Thiophosphoramidate Morpholino Oligonucleotides." Previously, in 2016, Paul and Caruthers showed that synthesizing phosphorodiamidate morpholinos (PMOs) and PMO-DNA chimeras is possible on DNA synthesizers using phosphoramidite chemistry (P.S. The paper was retracted). In this approach boronephosphoromidate morpholino internucleotide linkages are prepared first followed by oxidative substitution with different amines.
Langner et al. assumed phosphoramidates with P=S moieties will have improved hydrolytic stability under acidic conditions and increased cell nuclease resistance. The research group reported the optimized chemical synthesis of a new oligonucleotide analog called thiophosphoramidate morpholinos (TMOs). The combination of two well-studied pharmacophores, phosphorothioates (pS) and morpholinos, allowed the creation of morpholino–pS hybrid oligonucleotides. The newly simplified synthesis strategy enabled the incorporation of morpholino–pS moieties and sugar modifications in tandem to create this novel oligonucleotide (ON) analog. The research group used these new nucleic acid building blocks to synthesize TMO chimeras.
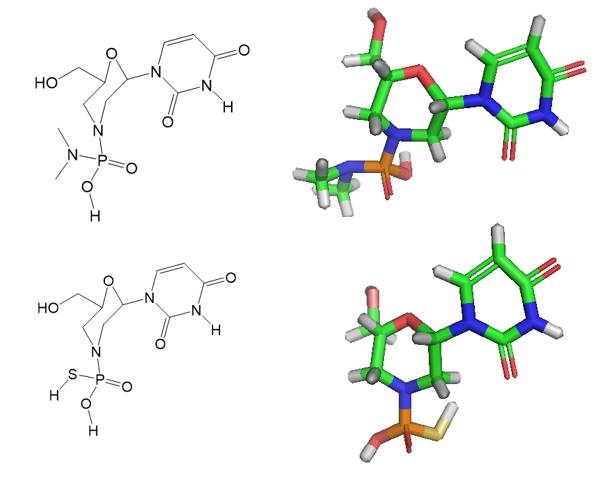
Figure 2: Building blocks for PMOs and TMOs.
Hybridization studies showed that TMO chimeras consisting of alternating TMO and DNA–pS subunits exhibited a higher binding affinity toward complementary RNA when compared to the canonical DNA/RNA duplex (∼10 °C).
Oligonucleotides that consist entirely of TMOs also showed a higher RNA binding affinity but did not recruit ribonuclease H1 (RNase H1). Also, chimeric TMO analogs showed a high gene silencing efficacy during in vitro bioassay screens used to evaluate their potential as microRNA inhibitors of hsa-miR-15b-5p in HeLa cells.
Next, Le et al., in 2022, introduced TMOs as splice-switching oligonucleotides. The research group designed and tested a synthetic antisense TMO called TMO1 ASO targeting exon 23 in the mouse dystrophin transcript. TMO1 induced exon skipping when transfected without lipofectin or nucleofection, demonstrating the ability of TMO1 to induce exon skipping in an in vitro model.
Reference
Bill, B. R., Petzold, A. M., Clark, K. J., Schimmenti, L. A., & Ekker, S. C. (2009). A primer for morpholino use in zebrafish. Zebrafish, 6(1), 69–77. [PMC]
Gentsch GE, Spruce T, Monteiro RS, Owens NDL, Martin SR, Smith JC. Innate Immune Response and Off-Target Mis-splicing Are Common Morpholino-Induced Side Effects in Xenopus. Dev Cell. 2018;44(5):597-610. [PMC]
Gerety SS, Wilkinson DG. Morpholino artifacts provide pitfalls and reveal a novel role for pro-apoptotic genes in hindbrain boundary development. Dev Biol. 2011 Feb 15;350(2):279-89. [PMC]
Heera K. Langner, Katarzyna Jastrzebska, and Marvin H. Caruthers; Synthesis and Characterization of Thiophosphoramidate Morpholino Oligonucleotides and Chimeras. J. Am. Chem. Soc. 2020, 142, 38, 16240–16253. [JACS]
Le BT, Paul S, Jastrzebska K, Langer H, Caruthers MH, Veedu RN. Thiomorpholino oligonucleotides as a robust class of next generation platforms for alternate mRNA splicing. Proc Natl Acad Sci U S A. 2022 Sep 6;119(36). [PMC]
Sibasish Paul and Marvin H. Caruthers; Synthesis of Phosphorodiamidate Morpholino Oligonucleotides and Their Chimeras Using Phosphoramidite Chemistry. J. Am. Chem. Soc. 2016, 138, 48, 15663–15672. [JACS]
Tumor protein p53 (Tp53)
---...---