Multidrug-resistant gram-negative bacteria are a serious and growing risk to public health. Gram-negative bacteria can cause antibiotic-resistant infections, often leading to death. Resistance to the antibiotic amikacin and other aminoglycosides in clinical settings is often due to the enzymatic acetylation of the antimicrobial molecule. In antibiotic-resistant Gram-negative bacteria, the enzyme 6’-N-acetyltransferase type Ib [AAC(6’)-Ib] mediates resistance to amikacin. This enzyme catalyzes the transfer of an acetyl group from acetyl coenzyme A to the 6’ position of the antibiotic molecule using acetyl-CoA as a donor substrate.
Magallon et al. recently observed that adding Zn2+ to in-vitro enzymatic reactions obliterated acetylation of the acceptor antibiotic. When added to amikacin-containing culture medium in complex with ionophores such as zinc pyrithione, it prevents the growth of resistant strains.
The anti-fungal drug zinc pyrithione is widely used in medical shampoos to treat dandruff and seborrheic dermatitis. Zinc pyrithione is a solid coordination complex of zinc with a molecular weight of 317.7 g/mol and a logP = 0.88. The bioactive monomeric molecule is the anti-fungal agent.
Magallon's research group in Tolmaky's lab found that a zinc pyrithione based inhibitor helps fight bacterial antibiotic resistance. More recently, in 2022, the researchers showed that water-soluble pyrithione compounds allow the design of inhibitors to fight bacterial resistance to the aminoglycoside amikacin.
Inhibiting the acetylation reaction will again render the antibiotic active against Gram-negative bacteria. To enable further use of amikacin against antibiotic resistant infections, inhibitors of the inactivating reaction can be combined with the antibiotic. Magda et al. previously derivatized pyrithione with the amphoteric group di(ethylene glycol)methyl ether at position 5 to increase its water solubility.
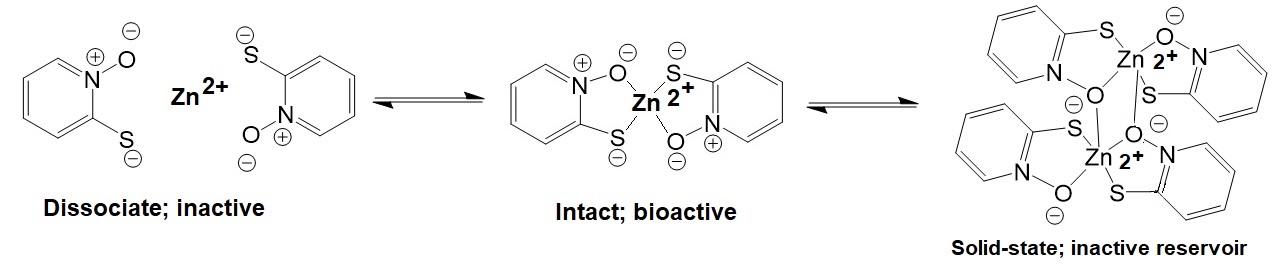
Figure 1: Chemical structures of zinc pyrithione.
Amikacin is a 4,6-linked aminoglycoside modified at position N1 of the 2-deoxystreptamine ring by the (l)-α-hydroxy-γ-aminobutyric amide (L-HABA) group. Amikacin binds specifically to the A site like its parent compound, kanamycin. The introduction of the L-HABA group on ring II of the aminoglycoside increases the affinity to the bacterial A site. Aminoglycoside antibiotics target the 16S ribosomal RNA (rRNA) bacterial A site where they induce misreading of the genetic code.
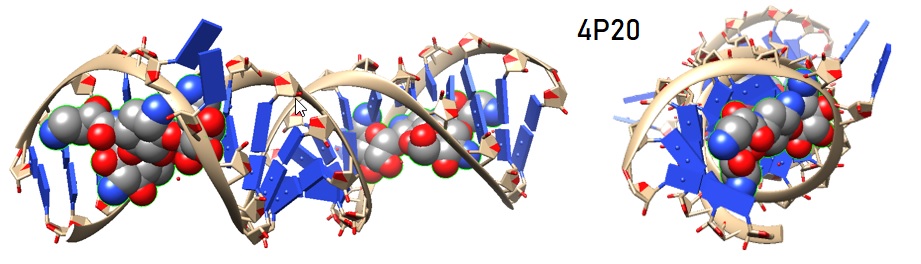
Figure 2: Structure of the bacterial ribosomal decoding site containing the bacterial ribosomal A site in complex with amikacin [Kondo et al. 2006. PDB ID 4P20].
One prominent mechanism of antibiotic resistance is the enzymatic modification of the active compound. The change prevents its binding to the cellular target. One common mechanism is N-acetylation at the 6’-position. The aminoglycoside 6’-N-acetyltransferase (AAC(6')) catalyzes the reaction. This enzyme's two functional classes have been described: AAC(6′)-I, which confers resistance to amikacin but not to gentamicin, and AAC(6′)-II, with reciprocal selectivity. Both enzyme classes also acetylate kanamycin, tobramycin, neomycin, netilmicin, and sisomicin. Some isoforms of the AAC(6')-Ib subclass also modify amikacin and gentamicin and potentially some fluoroqinolones.
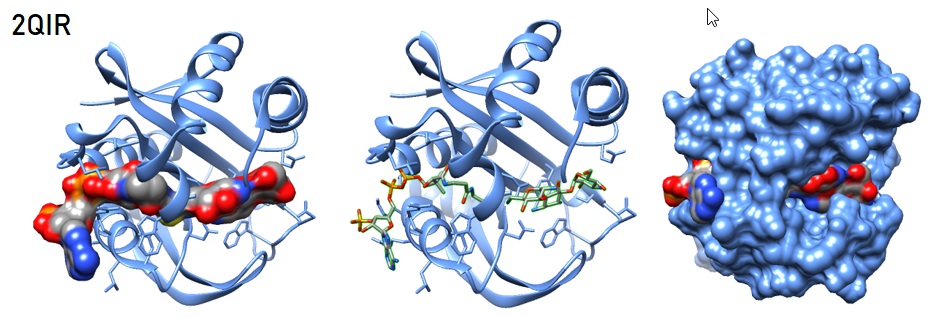
Figure 3: Structural model of aminoglycoside acetyltransferase AAC(6’)-Ib in complex with coenzyme A and kanamycin [Maurice et al. 2008. PDB ID 2QIR].
In 2013 Tada et al. observed that bacteria that acquire the aac(6′)-Iaj gene gain resistance to all aminoglycosides tested except to gentamicin. Thin-layer chromatography revealed that AAC(6′)-Iaj acetylated all tested aminoglycosides except gentamicin. The study found that AAC(6′)-Iaj is a functional acetyltransferase that modifies the amino groups at the 6′ positions of aminoglycosides and contributes to aminoglycoside resistance of P. aeruginosa NCGM1588, including arbekacin.
Kanamycin binds to four nucleotides of 16S rRNA and a single amino acid of protein S12 which interferes with the decoding site in the vicinity of nucleotide 1400 in 16S rRNA of the 30S subunit. This region interacts with the wobble base in the anticodon of tRNA leading to interference with the initiation complex resulting in misreading of mRNA so that incorrect amino acids are inserted into the polypeptide. The result is the synthesis of nonfunctional or toxic peptides and the breakup of polysomes into nonfunctional monosomes.
Synthesis of water soluble zinc pyrithione (according to Magda et al. 2008).
[1] Bromination of 2-Bromo-5-methyl-pyridine under efflux for 8 hours.
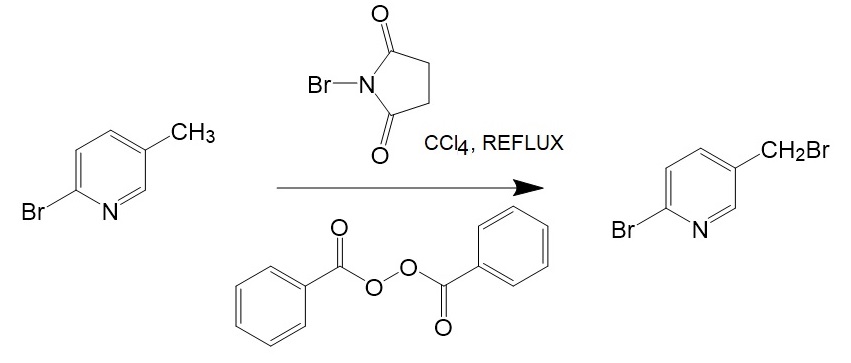
[2] Followed by PEGylation of 2-Bromo-5-bromomethylpyridine.
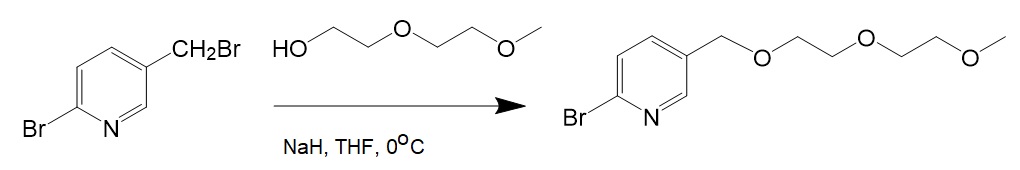
[3] Oxidation of 2-Bromo-5-CH2OPEG-pyridine.
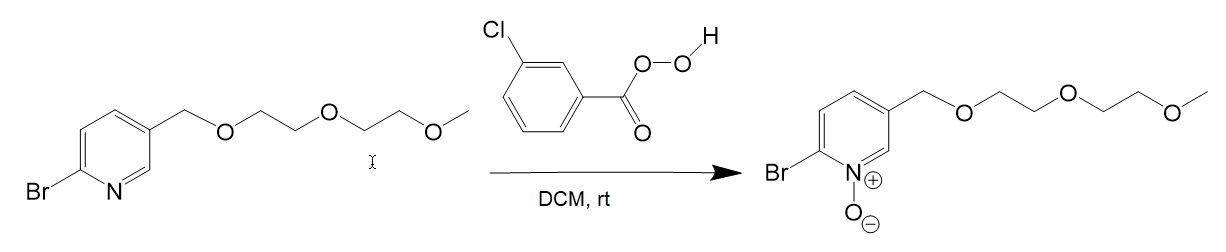
[4] Sodium sulfide reaction with 2-Bromo-5-CH2PEG-pyridine-N-Oxide.
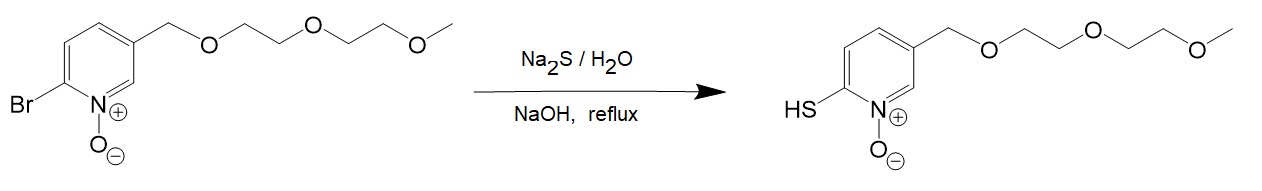
[5] Preparation of zinc complex of 2-mercapto-5-CH2PEG-pyridine-N-Oxide..
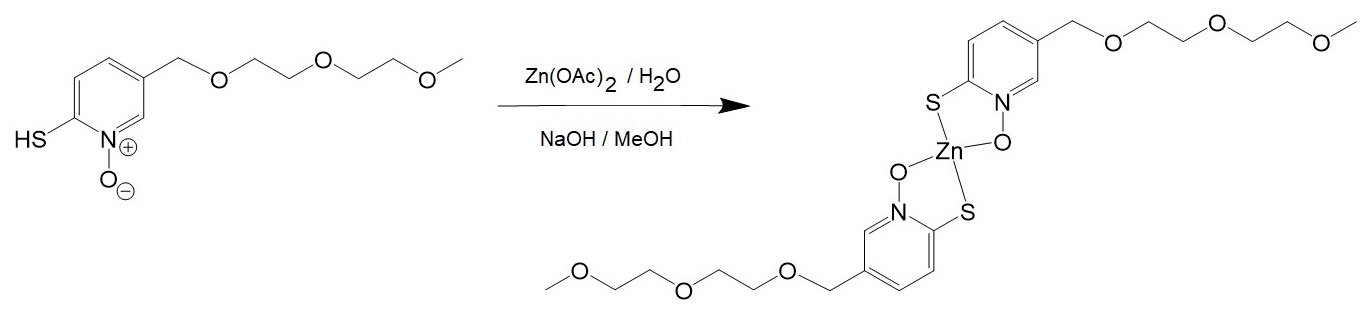
Time-kill assays performed by Magallon et al. showed that the addition of amikacin to bacterial cell cultures resistant to amikacin together with the water-soluble ionophore zinc pyrithione inhibited bacterial growth.
Reference
Amikacin
Kanamycin
Kondo J, François B, Russell RJ, Murray JB, Westhof E. Crystal structure of the bacterial ribosomal decoding site complexed with amikacin containing the gamma-amino-alpha-hydroxybutyryl (haba) group. Biochimie. 2006 Aug;88(8):1027-31. [PubMed]
Magallon J, Vu P, Reeves C, Kwan S, Phan K, Oakley-Havens CL, Rocha K, Jimenez V, Ramirez MS, Tolmasky ME. Amikacin potentiator activity of zinc complexed to a pyrithione derivative with enhanced solubility. Sci Rep. 2022 Jan 7;12(1):285. [PMC]
Magda D, Lecane P, Wang Z, Hu W, Thiemann P, Ma X, Dranchak PK, Wang X, Lynch V, Wei W, Csokai V, Hacia JG, Sessler JL. Synthesis and anticancer properties of water-soluble zinc ionophores. Cancer Res. 2008 Jul 1;68(13):5318-25. [Cancer Research]
Mangion SE, Holmes AM, Roberts MS. Targeted Delivery of Zinc Pyrithione to Skin Epithelia. Int J Mol Sci. 2021 Sep 8;22(18):9730. [PMC]
Maurice F, Broutin I, Podglajen I, Benas P, Collatz E, Dardel F. Enzyme structural plasticity and the emergence of broad-spectrum antibiotic resistance. EMBO Rep. 2008 Apr;9(4):344-9. doi: 10.1038/embor.2008.9. [PMC]
Reeves CM, Magallon J, Rocha K, Tran T, Phan K, Vu P, Yi Y, Oakley-Havens CL, Cedano J, Jimenez V, Ramirez MS, Tolmasky ME. Aminoglycoside 6'-N-acetyltransferase Type Ib [AAC(6')-Ib]-Mediated Aminoglycoside Resistance: Phenotypic Conversion to Susceptibility by Silver Ions. Antibiotics (Basel). 2020 Dec 31;10(1):29. [PMC]
Tada T, Miyoshi-Akiyama T, Shimada K, Shimojima M, Kirikae T. novel 6'-n-aminoglycoside acetyltransferase AAC(6')-Iaj from a clinical isolate of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013 Jan;57(1):96-100. doi: 10.1128/AAC.01105-12. [PMC]
---...---
Bio-Synthesis provides a full spectrum of bio-conjugation services including high quality custom oligonucleotide modification services, back-bone modifications, conjugation to fatty acids and lipids, cholesterol, tocopherol, peptides as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---