How to select the best purification method to purify oligonucleotides?
Selection of the optimal method for the purification of oligonucleotides depends on the following three major factors:
- Oligonucleotide length

- Application
- Modifications
[1] The first factor is the length of an oligonucleotide. The longer an oligonucleotide sequence is, the more failing sequences are potentially present in the crude mixture. Regardless of coupling efficiency, long oligonucleotides of greater than 50 bases are highly recommended to be purified.
[2] The second factor is application. For example, some oligonucleotides require characterization by ion exchange chromatography to achieve extremely high purity greater than 98%. This is important for cell viability. The preferred method of purification is anion exchange HPLC due to this ability to produce high quality oligos in the cell friendly Na+ salt form.
[3] The third factor is modifications. Sometimes a labeled or dual labeled oligonucleotide is required for the synthesis of a molecular beacon, for FRET, biotin-streptavidin, or bio-conjugation applications. In these special cases, the hydrophobicity of the modifying group at either the 5’- or 3’-end can be utilized to produce high quality oligos through C18 reverse phase HPLC.
The most common purification techniques used at Biosynthesis are:
-
Polyacrylamide gel electrophoresis (PAGE)
-
Reverse Phase High Pressure Liquid Chromatography (RP HPLC)
-
Anion Exchange High Pressure Liquid Chromatography (AEX HPLC)
Each purification technique has its own strengths and weaknesses. However, if done properly each purification method can produce high quality oligonucleotides.
PAGE:
Polyacrylamide gel electrophoresis is the oldest technique in oligonucleotide purification. Generally, a high percentage acrylamide gel is used to separate the full length products from all failed sequences based on their charge and ability to “run” through the gel matrix. PAGE can be used to separate oligos with lengths greater than 15 bases all the way up to 400 base pairs yielding purities greater than 90%. The advantage of PAGE is that it is the only purification technique that can resolve unmodified oligos of lengths greater than 80 bases. Biosynthesis always recommends long oligonucleotides greater than 80 bases to be purified by PAGE. However, there is one drawback. PAGE purified products will yield a lower final purified product compared to HPLC because it is impossible to recover every bit of full length product from the gel slice. However, the loss in final product is an acceptable tradeoff if high quality, unmodified long oligonucleotides are needed.
Shown below in Figure 1 is an analytical electropherogram of a 218 base oligonucleotide made and purified by Biosynthesis.
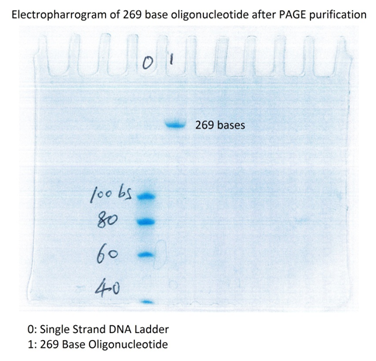
Figure 1: Analytical electropherogram of a PAGE purified unmodified 269 base oligonucleotide. The band clearly shows that all truncated sequences were removed.
RP HPLC:
Reverse phase is generally the “go-to” standard for HPLC purification of oligonucleotides due to its ability for quick mass spec and analytical HPLC quality control of fractions. General knowledge of how RP HPLC works is important to understanding the purification efficiency of RP HPLC. Many find it interesting that RP HPLC actually separates failed oligos from full length products by charge. How is it that a technique based on hydrophobicity of a compound can separate a polar molecule? In reality, RP HPLC of oligonucleotides is actually “ion-pairing chromatography”. This is a technique in which a long-chained alkyl amine is added in low concentration to the mobile phase in order to achieve enhanced resolution. Below is a schematic (scheme. 1) of how an oligonucleotide interacts with in ion-pairing reagent which in turn interacts with the C18 stationary phase of the column.
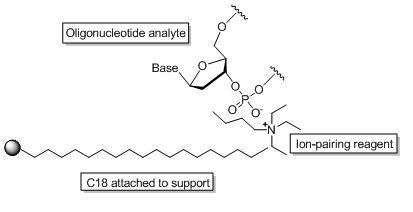
Scheme 1. Interaction of an oligonucleotide with an ion-pairing reagent at the surface of a C18 support.
This “indirect” interaction is the reason why RP HPLC can only resolve oligos from 8 to 40 bases with greater than 90% purity. However, if greater purity is required, the hydrophobic DMT group of the oligo can be left on the last 5’ base during synthesis, producing greater than 95% pure oligonucleotides. The advantage of both forms of HPLC (RP and AEX) over PAGE is that the yield is typically much higher relative to the starting scale.
A second advantage to reverse phase chromatography is its ability to resolve modified oligos from unmodified oligos. Typically, modifications at the 5’ or 3’ end of an oligonucleotide such as fluorophores, biotin, amino,sulfohydryl, etc. are hydrophobic enough to interact directly with the stationary phase of the C18 column. This leads to highly purified custom modified oligos that can be greater than 95% pure. The two disadvantages to RP HPLC are(unmodified) oligos greater than 40 bases and less than 8 bases cannot be resolved.
It is important to point out that RP HPLC purified oligonucleotides are left in the ion-pairing reagent salt form. Normally, this is the triethylammonium amine (TEAA) salt. If the oligonucleotide is to be used in living systems such cells or animals, TEAA is very toxic. A simple sodium salt exchanged can be performed to remove any TEAA that would cause harm to cells.
Shown below in fig. 2, fig. 3, and fig. 4 is a preparatory chromatogram,mass spectra, and analytical report, respectively, of a 9mer 3’ Atto 655 modified oligonucleotide that was made and purified by Biosynthesis.
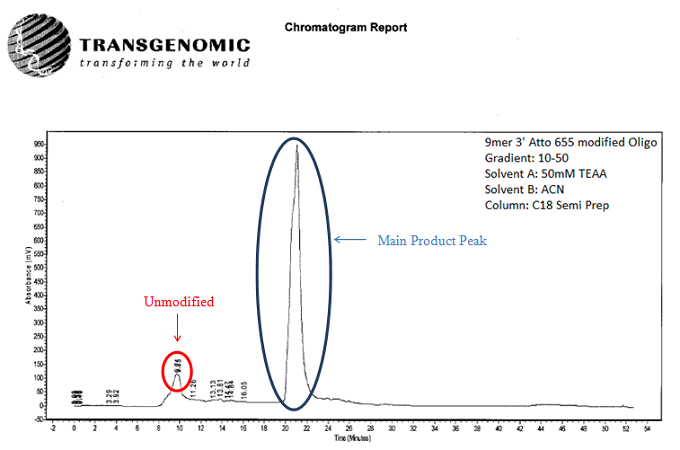
Fig 2. Semi-preparatory RP HPLC chromatogram of a crude 9mer 3’ Atto 655 modified oligonucleotide. The peak at 9.85 minutes represents the unmodified oligonucleotide. The main peak represents the highly pure Atto 655 modified product.
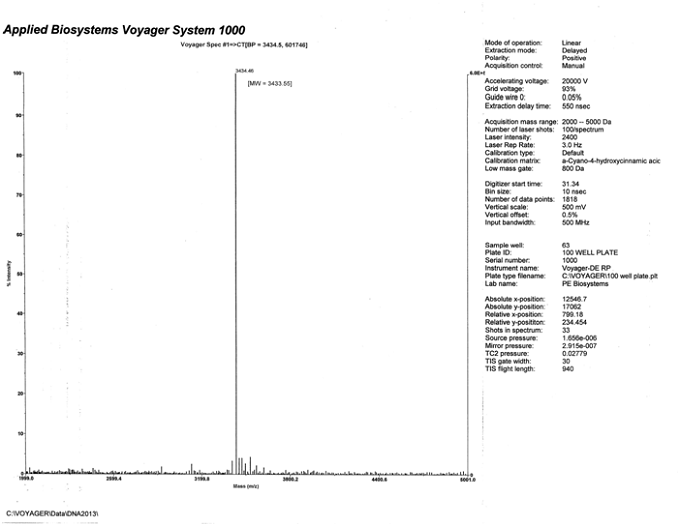
Fig 3. Mass Spectra of main peak collected from preparatory RP HPLC of the 9mer 3’Atto 655 modified oligonucleotide. The calculated molecular weight is 3433.55. The observed molecular weight is 3434.46.
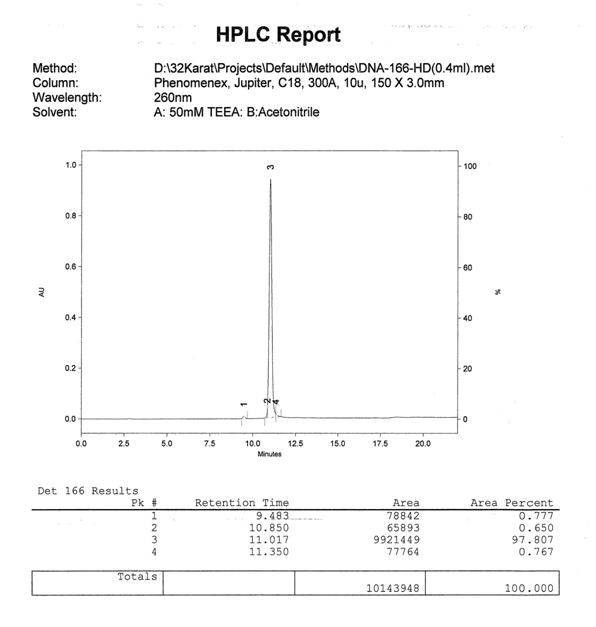
Fig 4. Analytical HPLC report of 9mer 3’ Atto 655 modified oligonucleotide of main peak from preparatory RP HPLC. Fluorophores such as Atto 655 contain enough hydrophobicity to allow for excellent reverse phase separation yielding high purity oligonucleotides.
AEX HPLC:
Anion-exchange chromatography is an established technology with a long history. AEX HPLC is the best form of purification for unmodified oligonucleotides up to 80 bases. The difference between AEX HPLC purification and RP HPLC purification is the way oligonucleotides interact with the stationary phase. In RP HPLC, the interaction was indirect. However, in AEX HPLC the negatively charged phosphate backbone of the oligonucleotide can directly interact with the positively charged stationary phase of the anion exchange column. This results in higher a resolution.
There are 3 main advantages to AEX HPLC. The first advantage is that oligomers up to 50 bases can be greater than 95% pure. From 50 to 80 bases, 95% or greater purity can also be achieved, but at lower yields. The second advantage is that AEX HPLC is the only type of purification technique that can successfully separate phosphothiolate, LNA, and BNA modified oligonucleotides. Generally, Biosynthesis recommends AEX HPLC when these modifications are present. However, if a hydrophobic modifying group is present, RP HPLC can also be used. The last advantage is that oligos that elute from an AEX HPLC system are in the Na salt form ready for use in living cells. The only minor setback of AEX HPLC is its slightly higher cost compared to RP HPLC and its lower resolving capability of 3’ or 5’ modifiedoligos. In such cases many customers chose DUAL HPLC purification with both RP and AEX HPLC to achieve 98% or greater purity.
Shown below in Fig. 5, Fig. 6, and Fig. 7 is a preparatory chromatogram, mass spectra, and analytical report respectively of a 28mer oligonucleotide purified by Biosynthesis.
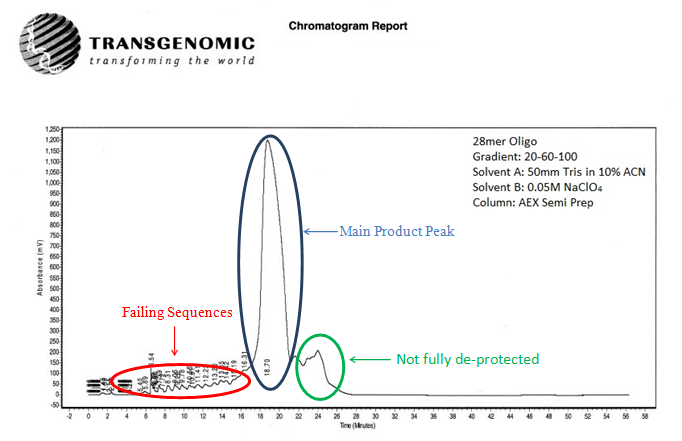
Figure 5: Semi-preparatory AEX HPLC chromatogram of a crude 28mer oligonucleotide. Failure sequences are represented as peaks before the main peak. The small peaks after the main peak are sequences that are not fully de-protected.
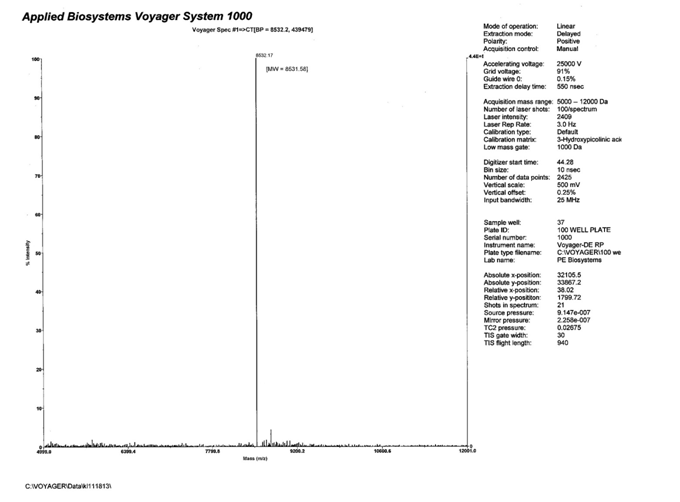
Figure 6: Mass Spectra of main peak collected from preparatory AEX HPLC of the 28mer unmodified oligonucleotide. The calculated molecular weight is 8531.58. The observed molecular weight is 8532.17.
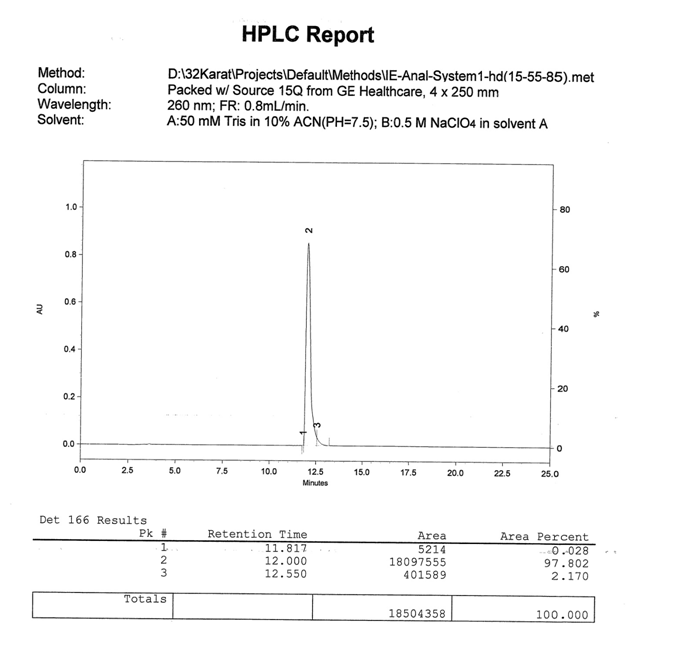
Figure 7: Analytical AEX HPLC report of the unmodified 28mer oligonucleotide from the main peak of the preparatory AEX HPLC. Purities exceeding 95% in this case 97.8% are commonly obtained when using AEX HPLC to purify oligonucleotides.
Whatever purification methods our customers decide to choose, Biosynthesis is committed to synthesizing the purest oligonucleotides.
-.-