Proximity ligation assay utilizing fluorescently labeled oligonucleotide probes allows the detection of protein-protein interactions implicated in Alzheimer’s disease (ApoE-C1q) and breast cancer (ErbB3-EGFR)
Within cells, a number of biological processes depend on protein-protein interaction. The interaction could be either stable or transient depending on the nature of function it assumes. For instance, a stable interaction of RNR1 and RNR2 subunits is required to form an active ribonucleotide reductase enzyme, which catalyzes the conversion of ribonucleotides to deoxyribonucleotides for DNA synthesis. Among the strongest binding types is the interaction between RNase A and ribonuclease inhibitor, which exhibits dissociation constant (KD) in the femtomolar (10-15) range. In contrast, the receptors involved in signal transduction interact transiently as the binding is reversible (Perkins et al., 2010). An exemplary case is the ErbB family receptor, which undergoes conformational change (upon binding to a ligand) to dimerize with distinct ErbB receptor (Macdonald-Obermann et al.., 2013).
It follows that the ability to detect protein-protein interaction could be highly useful to both basic science research as well as translational medicine. A commonly used technique for in vitro application is based on affinity purification followed by mass spectrometry analysis of associating proteins for identification. Alternatively, co-immunoprecipitation assay could be performed on cell extract using an antibody directed against the target protein to isolate its interacting proteins. For in vivo detection, a ‘two-hybrid’ screening method can be employed, wherein the interaction between two separate proteins expressed by distinct plasmids leads to the transactivation of a reporter gene in transfected cells. The latter method was used to uncover the interaction between the C-terminus and N-terminus of Rb (retinoblastoma) protein (Hensey et al., 1994_).
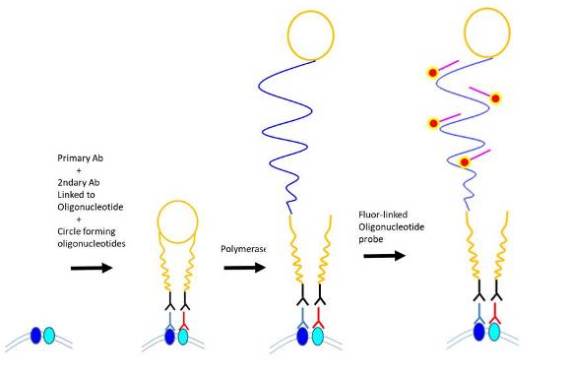
Proximity ligation assay (PLA) represents an immunological method for detecting protein-protein interactions with a single molecule resolution (Soderberg et al., 2006). In addition to the high degree of sensitivity and specificity, PLA could be used to confirm the protein-protein interaction detected by co-immunoprecipitation or co-localization assay in a short period of time. To detect, two distinct primary antibodies are used to bind to each interacting protein separately. Subsequently, it is bound to secondary antibody conjugated to unique oligonucleotides. If the two interacting proteins are proximally located (not necessarily juxtaposed), then the two oligonucleotides can form a circular DNA strand in the presence of two additional circle-forming oligonucleotides. After ligating to form a closed circle, polymerase is added to extend via rolling-circle DNA synthesis. By hybridizing fluorescently labeled oligonucleotides to the “amplified” DNA, the protein-protein interaction within cells can be detected by fluorescence microscopy (Zatloukal et al., 2014).
Alzheimer’s disease is a neurodegenerative disease affecting >5 million in the U.S and >29 million individuals globally. It accounts for 60-70% of dementia and negatively impacts memory, speech, mood, behavior, body function, etc. with the life expectancy of 3-9 years following diagnosis. Though most cases are sporadic, genetic analysis of hereditary cases has identified several genes that are associated with the early onset of Alzheimer’s disease. Amyloid beta peptide (ABP) is responsible for the plaque formation in the brain, which is derived through the cleavage of amyloid precursor protein encoded by APP gene (Findeis, 2007). Apolipoprotein E (ApoE) represents another gene linked to Alzheimer’s disease as ApoE binds to ABP and may affect ABP aggregation or clearance from the brain (Holtzman et al., 2012).
More recently, the role of APoE, which is also implicated in atherosclerosis (narrowing of arteries due to plaque buildup), has been investigated from an alternate perspective. Classical complement cascade (CCC) refers to an immunological mechanism that functions to activate phagocytosis, promote inflammation, and perforate cell membrane to eliminate invading microbes. Intriguingly, the investigators at Ludwig-Maximilians-University (Germany) have found that CCC is activated in ApoE deficient mice. Consistently, ApoE was shown to suppress CCC activity by binding to C1q protein, which initiates the CCC process (Yin et al., 2019). To demonstrate the latter, they used proximity ligation assay (PLA) to detect C1q-ApoE complexes formed in cultured human cells, which was visualized by fluorescence microscopy.
ErbB/HER family is comprised of 4 cell membrane-associated growth factor receptors necessary for normal mammary gland development. However, Her-2 receptor is overexpressed in 20-30% of breast cancer and it prognosticates a poor outcome. Recent works suggest that the dimerization of Her-3 (ErbB3) receptor with other HER family members may lead to the activation of Her-2. Using proximal ligation assay, Her-3’s interaction with Her-1 (epidermal growth factor receptor, EGFR) was detected in breast cancer specimens (Karamouzis et al. 2015).
Bio-Synthesis, Inc. specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology that we offer provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power was especially useful for diagnosis (ex. FISH using DNA probe). More importantly, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides for clinical application.
https://www.biosyn.com/oligonucleotide-modification-services.aspx
https://www.biosyn.com/tew/fluorescent-labeling-of-oligonucleotides.aspx
https://www.biosyn.com/oligo-flourescent-labeling.aspx
References
Findeis MA. The role of amyloid beta peptide 42 in Alzheimer's disease. Pharmacol Ther. 116:266-86. (2007). PMID: 17716740 DOI: 10.1016/j.pharmthera.2007.06.006
Hensey CE, Hong F, Durfee T, Qian YW, Lee EY, Lee WH. Identification of discrete structural domains in the retinoblastoma protein. Amino-terminal domain is required for its oligomerization. J Biol Chem. 269:1380-7 (1994). PMID:8288605 https://www.jbc.org/content/269/2/1380.long
Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2:a006312 (2012). PMID:22393530 doi: 10.1101/cshperspect.a006312.
Karamouzis MV, Dalagiorgou G, Georgopoulou U, Nonni A, Kontos M, Papavassiliou AG. HER-3 targeting alters the dimerization pattern of ErbB protein family members in breast carcinomas". Oncotarget. 7: 5576–97 (2016). PMC 4868707. PMID 26716646. doi:10.18632/oncotarget.6762.
Macdonald-Obermann JL, Adak S, Landgraf R, Piwnica-Worms D, Pike LJ. Dynamic analysis of the epidermal growth factor (EGF) receptor-ErbB2-ErbB3 protein network by luciferase fragment complementation imaging. J Biol Chem. 288:30773-84 (2013). PMID: 24014028 doi: 10.1074/jbc.M113.489534. Epub 2013 Sep 6.
Perkins JR, Diboun I, Dessailly BH, Lees JG, Orengo C. Transient protein-protein interactions: structural, functional, and network properties. Structure 18:1233-43 (2010) PMID: 2094701 doi: 10.1016/j.str.2010.08.007.
Söderberg O, Gullberg M, Jarvius M, Ridderstråle K, Leuchowius K, Jarvius J, et al. Direct Observation of Individual Endogenous Protein Complexes in Situ by Proximity Ligation. Nat Methods 3, 995-1000 (2006). PMID: 17072308 DOI: 10.1038/nmeth947
Yin C, Ackermann S, Ma Z, Mohanta SK, Zhang C, Li Y, Nietzsche S, Westermann M, et al. ApoE attenuates unresolvable inflammation by complex formation with activated C1q. Nat Med. 25:496-506 (2019). PMID: 30692699 doi: 10.1038/s41591-018-0336-8.
Zatloukal B, Kufferath I, Thueringer A, Landegren U, Zatloukal K, Haybaeck J. Sensitivity and specificity of in situ proximity ligation for protein interaction analysis in a model of steatohepatitis with Mallory-Denk bodies. PLoS One. 9(5):e96690 (2014). PMID:24798445 doi: 10.1371/journal.pone.0096690. eCollection 2014.