Double-stranded small interfering RNA (siRNA) silences the expression of specific genes. siRNAs have demonstrated their potential in treating various diseases and are a powerful tool for research and developing new therapies. However, siRNA can also interact with the immune system in several ways. Therefore, it is critical to understand the potential risks and benefits of using siRNA to modulate the immune system before using it in any therapeutic setting.
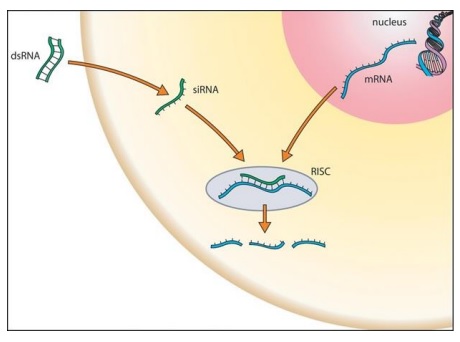
Figure 1: Molecular mechanism of RNA interference (RNAi). The process in which RNA molecules activate the cellular response to destroy specific RNA molecules such as messenger RNAs (mRNAs). (Source: Wiki Commons).
RNA interference (RNAi) therapies specifically target genes of interest. As more biological data have become available, it became apparent that in addition to mediating RNAi, siRNA molecules also have the potential to induce the innate immune system.
One significant challenge when designing siRNAs is the differentiation between therapeutic effects and non-specific innate immune system stimulation. Well-designed and considered experimental measures to establish the best design of siRNAs may avoid activating the immune system by siRNA molecules.
RNAi is an endogenous cellular mechanism by which sequence-specific siRNA induces gene silencing by targeting and cleavage of complementary messenger RNA (mRNA) within the cell's cytoplasm. The presence of double-stranded RNA (dsRNA) triggers RNAi. dsRNA is cleaved by the intracellular enzyme dicer into 21-base pair fragments of siRNA. Loading the siRNA into a protein complex called the RNA-inducing silencing complex (RISC) unwinds the siRNA and retains the antisense strand. RISC searches for any mRNA complementary to the antisense strand and cleaves it. As a result, the target gene is silenced and prevented from protein production.
Double-stranded RNA, when longer than 30 base pairs, can also be a potent activator of the innate immune interferon (IFN) response. Long dsRNA is a hallmark of viral infection. This response can limit siRNA therapies since siRNA delivery into cells introduces a foreign material into the cell's biological system. However, synthetically designed siRNA containing modified nucleic acids can circumvent Dicer mechanics and immunostimulation associated with long dsRNA.
The innate immune system is the body's first defense against infection and injury. The innate immune system cells quickly recognize and respond to various pathogens and danger signals. The mammalian immune system recognizes siRNA as a signature of viral infection; hence, siRNA can induce a potent and potentially dangerous innate immune response.
siRNA potentially activate the innate immune system in different ways.
[1] Toll-like receptors (TLRs) can recognize and bind siRNA. TLRs are proteins located on the surface of immune cells that recognize various danger signals.
[2] Other immune sensors, such as retinoic acid-inducible gene I (RIG-I), can bind siRNA.
When siRNA activates the innate immune system, it can trigger several different responses, including the production of inflammatory cytokines and the activation of immune cells. However, using siRNA to treat infection can induce a beneficial immune response activation. Sometimes, a harmful activation can occur when treating a chronic disease with siRNA.
[3] Another way siRNA interacts with the immune system is through the adaptive immune system. The adaptive immune system is a more sophisticated defense system that learns to recognize and respond to specific pathogens. It comprises B and T cells, which produce antibodies and other immune molecules that can neutralize or kill pathogens.
In addition, siRNA also enables modulation of the adaptive immune system. For example, siRNA allows silencing the expression of genes involved in activating or suppressing T cells. siRNA can also deliver genes to T cells that enhance their anti-tumor activity.
The mammalian immune system can recognize siRNA as a foreign molecule, likely a sign of viral infection, and respond robustly. A list of heterogeneous pattern recognition receptors (PRR) in different cell types of the mammalian system is shown in Table 1.
Table 1: Pattern recognition receptors (PRRs) that recognize and respond to RNA.
|
PRR
|
Ligand
|
Cell line expression
|
Subcellular location
|
Primary response
|
|
TLR3
|
siRNA
dsRNA
|
Epithelial cells
Fibroblast
|
Cell surface
|
IFN-α
IFN-β
IFN-γ
|
|
Epithelial cells
Fibroblast
mDCs
|
Endosome
|
IFN-α
IFN-β
IFN-γ
|
|
TLR7
|
siRNA
ssRNA
|
pDCs
|
Endosome
|
IFN-α
IFN-β
|
|
Lysosome
|
TNF-α
IL-12
|
|
B cells
|
|
B cell differentiation
|
|
TLR8
|
siRNA
ssRNA
|
Monocytes
Macrophages
mDCs
|
Endosome
|
IFN-α
IFN-β
|
|
Lysosome
|
TNF-α
IL-1
IL-6
IL-12
|
|
PKR
|
Long dsRNA
|
Most mammalian cells
|
Cytoplasm
|
IFN-α
IFN-β
Inhibition of protein translation
|
|
RIG-I
|
Uncapped 5’-triphosphate RNA and blunt RNA
|
Fibroblasts
mDCs
|
Cytoplasm
|
IFN-α
IFN-β
|
Abbreviations: TLR, Toll-like receptor; siRNA, small interfering RNA; dsRNA, double-stranded RNA; IFN, interferon; mDCs, mature dendritic cells; ssRNA, single-stranded RNA; pDCs, plasmacytoid dendritic cells; TNF-α, tumor necrosis factor α; IL, interleukin; PKR, double-stranded RNA-dependent protein kinase; RIG-I, retinoic acid-inducible gene I protein.
Examples of immune-stimulating sequences
The nucleotide sequence of siRNAs can affect its immunostimulatory properties. Immunostimulation motifs of the TLR7-mediated interferon response, 5-UGU-3’, 5’-GUCCUUCAA-3’, induce cytokine production independent of the number of GU nucleosides. These are two examples of the many specific RNA sequences that TLR7 and 8 recognize. A ribose sugar backbone and multiple uridine residues near one another also stimulate TLR7 and 8. However, substituting guanosine with adenosine reduced TNF-α, and replacing uridine with adenosine decreased IFN-α production in plasmacytoid dendritic cells. Modifications to the 2’-position on the ribose ring of the RNA backbone can reduce innate immune activation. Also, the delivery vehicle selected to facilitate siRNA transfection potentially affects the innate immune response. As a result, researchers need to design delivery experiments for siRNA carefully.
The chemical structure of nucleic acids can influence the immune response
The chemical structure of the siRNA duplex can also influence how the innate immune system responds. For example, RIG-I can bind to ssRNA or dsRNA containing uncapped 5’-triphosphate groups, resulting in an interferon-mediated immune response. Uncapped RNA is a sign of viral infection causing a subsequently induced inflammatory action. Further, blunt-ended dsRNA can provoke an immunostimulatory activity after recognition by RIG-I. Incorporating 3’-overhangs on either or both RNA strands reduces these activities. Self microRNAs present in the cytoplasm processed by dicer include 3’-overhangs. However, viral dsRNAs typically possess blunt ends.
Chemical changes in the ribose backbone can reduce or even eliminate the innate immune response. For example, inserting bridged nucleic acids (BNAs/LNAs) into RNA strands can reduce immune recognition and response. Still, it can also compromise the potency of the siRNA depending on which point the BNA is inserted. The 2’-O-Me modification acts as a potent inhibitor of RNA-induced immune stimulation without diminishing RNAi potency.
Modulating the immune system
Examples of how siRNA can modulate the immune system:
siRNA potentially allows the development of new vaccines against cancer and infectious diseases.
siRNA may enable new treatments of autoimmune diseases, such as rheumatoid arthritis and multiple sclerosis.
siRNA may allow the development of new treatments for allergies and asthma.
siRNA potentially allows the development of new treatments for cancer by targeting genes that are involved in tumor growth and metastasis.
Reference
Kanasty RL, Whitehead KA, Vegas AJ, Anderson DG. Action and reaction: the biological response to siRNA and its delivery vehicles. Mol Ther. 2012 Mar;20(3):513-24. [PMC]
Suzuki M, Zheng X, Zhang X, Ichim TE, Sun H, Kubo N, Beduhn M, Shunnar A, Garcia B, Min WP. Inhibition of allergic responses by CD40 gene silencing. Allergy. 2009 Mar;64(3):387-97. [Allergy]
Spurgers KB, Sharkey CM, Warfield KL, Bavari S. Oligonucleotide antiviral therapeutics: antisense and RNA interference for highly pathogenic RNA viruses. Antiviral Res. 2008 Apr;78(1):26-36. [PMC]
Whitehead KA, Dahlman JE, Langer RS, Anderson DG. Silencing or stimulation? siRNA delivery and the immune system. Annu Rev Chem Biomol Eng. 2011;2:77-96. [PubMed]
Zhao Y, Huang L. Lipid nanoparticles for gene delivery. Adv Genet. 2014;88:13-36. [PMC]
Other links
https://www.biosyn.com/faq/What-are-Backbone-Modifications-in-Oligonucleotide-Synthesis.aspx#!
https://www.biosyn.com/backbone-modified-oligonucleotide-synthesis.aspx#
---...---
Bio-Synthesis provides a full spectrum of bio-conjugation services including high quality custom oligonucleotide modification services, back-bone modifications, conjugation to fatty acids and lipids, cholesterol, tocopherol, peptides as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs, miRNA, or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---