Tyramide Signal Amplification (TSA)
By Klaus D. Linse
Tyramide Signal Amplification is a novel technique using a biotinylated tyramine to detect specific proteins or nucleic acid sequences in-situ, where tyramide, a phenolic compound, has the ability to bind to the electron rich surface of targets. Signal amplification is the use of specific detection methodologies to directly increase the signal in proportion to the amount of target in a reaction by simultaneously minimizing the possibility of contamination by target amplification products. In general, a reporter group or enzyme is used for this type of reaction. For example, during gene amplification the number of gene copies is increased in cells. One powerful technique used in molecular biology is the polymer chain reaction (PCR) that amplifies target DNA allowing the production of increased quantities of DNA. Some commonly used signal amplification technologies include branched DNA (bDNA) and hybrid capture (HC) assays. Signal amplification is a common method used nowadays to amplify the target signal in in-situ hybridization assays, such as CARD-FISH, with the help of haptens. A hapten can be any small molecule that, when combined with a larger carrier such as a protein, induces the production of antibodies that specifically bind to it either in the free form or connected to carrier molecule.
The general principle of the CARD-FISH or TSA method is illustrated in Figure 1.
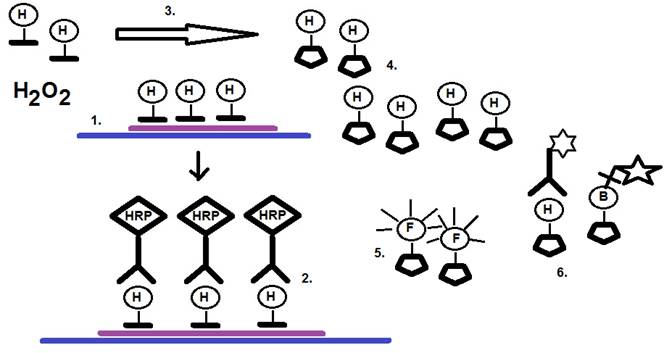

Figure 1: Principle of CARD-FISH or catalyzed reported deposition-fluorescence in-situ hybridization.
Legend:
H = hapten = biotin, digoxigenin, dinitrophenyl, fluorochrome-labled probe
HRP = horseradish peroxidase; hapten H = fluorochrome = F; B = biotin; stars = flurochrome or enzyme.
1. Hybridization in-situ involves the use of a hapten such as biotin, dioxigenin, dinitrophenyl, and or a fluorochrom-labeled probe.
2. The use of a probe covalently conjugated to horseradish peroxidase (HRP) such as an anti-hapten antibody or oligonucleotide.
3. CARD signal amplification is achieved with hapten-labeled tyramine or a probe labeled with a single flourochrome that enables the use of radicalization of multiple tyramide molecules and hydrogen peroxide.
4. Deposition of tyramide radicals to tyrosine moieties of proteins in-situ in the vicinity of the site of synthesis.
5. Direct visualization of flouorochrome-labled tyramines.
6. Indirect visualization of biotin-labeled or hapten-labeled tyramides with strepavidin, avidin or anti-hapten antibody conjugates labled with fluorochromes. In addition, the use of enzyme precipitation reactions allows the visualization of enzyme activities.
What is TSA or CARD?
Tyramide Signal Amplification, or TSA, sometimes also called Catalyzed Reporter Deposition, or CARD, or tyramine amplification technique (TAT), is an enzyme-mediated detection method that utilizes the catalytic activity of horseradish peroxidase (HRP) for the generation of a high-density labeled target protein or nucleic acid sequence in-situ. Signal amplification in TSA is enabled via binding of biotinylated tyramine to proteins near the site of peroxidase-labeled antibodies. This technique enables reliable detection and quantitation of proteins as well as nucleic acids. In comparison to conventional standard avidin-biotinylated enzyme complex (ABC) based assays, TSA has been reported to increase detection sensitivity up to 100-fold. In addition, TSA can be combined with several other technologies such as nucleic acid labeling, primary and secondary antibodies, avidin and lectin conjugates, cytoskeletal stains, organelle probes and cell tracers based detection techniques. TSA can be used to improve current immunohistochemistry, (IHC), immunofluorescence , (IF), or in-situ hybridization, (ISH), based protocols using existing imaging hardware, for example microscopes.
This amplification method is based on the characteristic ability of tyramine to become chemically sticky after oxidative radicalization. In TSA, HRP reacts with hydrogen peroxide and the phenolic part of tyramine to produce quinone-like structures that carry a radical on the C2 group. But first, the targeted epitope is detected with HRP with the help of specific antibodies. The incubation of the labeled tissue with biotinylated tyramine and hydrogen peroxide (H2O2) results in a peroxide enzyme catalyzed reaction that adds radicals to tyramine. Peroxidases catalyze dehydrogenation by hydrogen peroxide (H2O2) of various phenolic and endiolic substrates in a peroxidatic reaction cycle. Enols are alkenes containing a hydroxyl group connected to one of the carbon atoms at the double bond. Horseradish peroxidase (HRP) can also catalyze a third type of reaction that results in the production of hydroxyl radicals (·OH) from H2O2 in the presence of O2·-. The radicalized tyramine can now bind covalently to nearby tissue molecules, thereby amplifying the signal. The biotin on the bound tyramine serves as a tracer molecule that can be visualized using standard techniques that use avidin-biotin-enzyme complex formation reactions. The conjugation of tyramine molecules to a hapten or fluorochrome make the indirect and direct fluorescence detection of enzymatically deposited tyramides possible.
To illustrate the HRP reaction the conjugation of fluorescein-tyramide to a protein as catalyzed by peroxides is shown in Figure 2.
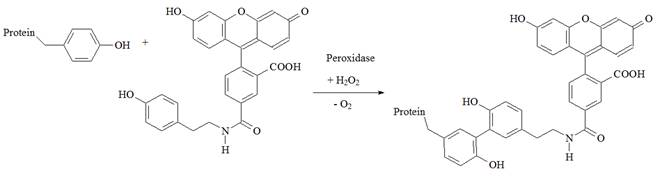
 Figure 2: Conjugation of fluorescein-tyramine to a protein. The fluorescein-tyramide is covalently linked to a protein tyrosine side chain via peroxidase-mediated formation of the dityrosine adduct.
Figure 2: Conjugation of fluorescein-tyramine to a protein. The fluorescein-tyramide is covalently linked to a protein tyrosine side chain via peroxidase-mediated formation of the dityrosine adduct.
Principles of Tyramide Signal Amplification
TSA labeling is a combination of several elementary chemical and enzymatic processes (See Figure 1).
- The selected probe is bound to the target using immunoaffinity or hybridization. Secondary detection of the probe is achieved with a HRP-labeled antibody or streptavidin conjugate. Peroxidase conjugates of other targeting proteins such as lectins and receptor ligands may also be suitable for labeling targets as well as endogenous peroxidase activity. In addition, unconjugated HRP can be used as a neuronal tracer often in combination with TSA.
- Multiple copies of a labeled tyramide derivative are activated with HRP. For this, fluorescent or biotinylated tyramide is used. In addition, other hapten-conjugated tyramides, tyramide-conjugated gold particles, as well as other similar molecules may be used.
- The covalent coupling of the resulting highly reactive, short-lived tyramide radicals to residues at the phenol moiety of protein tyrosine residues in the vicinity of the HRP-target interaction site in proximity to the target results in signal localization.
Applications of CARD or TSA
- Fluorescein-labeled tyramine can been used to detect protein oxidation by reactive oxygen species in tissue.
- Detection and quantification of low abundance analytes such as peptide, proteins, DNA or RNA molecules.
- Improving the performance of weakly binding primary antibodies.
- Improving of background by reducing the amount of antibodies or probes needed for the detection.
- Reduction of scanning times which results in the faster production of images.
- Mapping of DNA probes of less than 1 kb size.
References
Rudolf Amann and Bernhard M. Fuchs; Single-cell identification in microbial communities by improved fluorescence in-situ hybridization techniques. Nature Reviews 2008, Volume 6, 339-348.
L.M. Schriml, H.M. Padilla-Nash, A. Coleman, P. Moen1, W.G. Nash2, J. Menninger, G. Jones, T. Ried and M. Dean; Tyramide Signal Amplification (TSA)-FISH Applied to Mapping PCR-Labeled Probes Less than 1 kb in Size. BioTechniques 27:608-613 (September 1999).
Ernst J.M. Speel, Anton H.N. Hopman and Paul Komminoth; Amplification Methods to Increase the Sensitivity of In-Situ Hybridization: Play CARD(S). J Histochem Cytochem 1999, 47: 281.
Reinhard von Wasielewski, Michael Mengel, Suzanne Gignac, Ludwig Wilkens, Martin Werner and Axel Georgii; Tyramine Amplification Technique in Routine ImmunohistochemistryThe Journal of Histochemistry & CytochemistryVolume 45(11): 1455–1459, 1997.