Amino acids are stereo-isomers, posses handedness and are chiral molecules
Molecules with the same atoms and functional groups are called stereoisomers. Stereoisomers are compounds made up of the same atoms and bonded by the same sequence of bonds, but having different three dimensional (3D) structures. The different 3D structures are called configurations and are not interchangeable. Two stereoisomers cannot superimpose. Thus, even when two molecules contain the same functional groups but are stereoisomers organisms can usually distinguish between them.
Figure 1 illustrates the concepts of chirality, enantiomers, handedness, isomers and stereoisomers. The amino acid alanine is used here as an example.
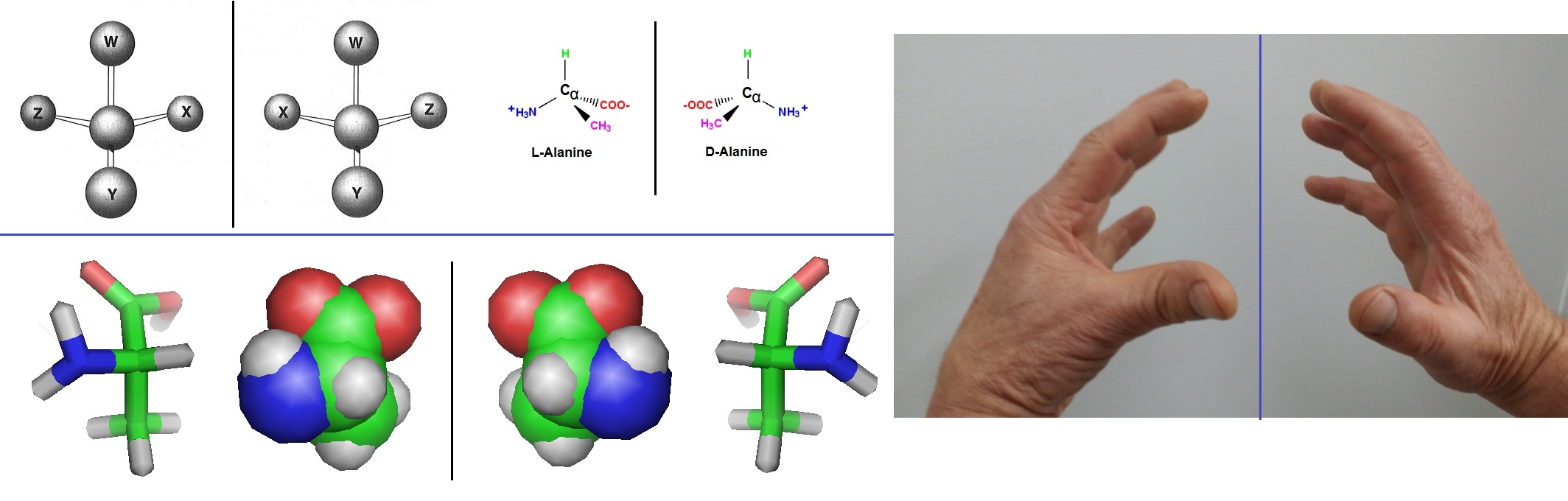
Figure 1: Enantiomers are illustrated on the left site of the figure. The right site illustrated handedness, which is the tendency to use one hand rather than the other, as well as the property of the two hand of not being identical with its mirror image. The property of nonsuperimposability of an object on its mirror image, in this case for a pair of hands and the enantiomers of the amino acid alanine, called chirality is illustrated here.
Any material that has the ability to rotate the plane of polarized light is known to be optically active. If a pure compound or molecule is optically active, the structure of the molecule is nonsuperimposable on its mirror image. On the other hand, if a molecule is superimposable on its mirror image, the compound does not rotate the plane of polarized light and it is optically inactive. The property of nonsuperimposability of an object on its mirror image is called chirality. If a molecule is superimposable on its mirror image it is optically inactive and called achiral. Apparently the the relationship between optical activity and chirality is absolute and no exceptions are known.
The term chirality as used in chemistry, biochemistry and biology describes the property of asymmetry molecules can posses. The word chirality is derived from the Greek word for “hand”, χειρ (kheir). Human hands are an example for a chiral object. An object or a system is chiral if it is not identical to its mirror image, that is, it cannot be superposed onto it.
If a molecule is nonsuperimposable on its mirror image, the mirror image must be a different molecule. Superimposability is the same as identity, thus the image and the mirror image correlates with the same molecule.
Pure compounds that are optical active have two and only two isomers. These are called enantiomers or sometimes enantiomorphs. The two enantiomers differ in structure only in the left and right handedness of their orientation.
Enantiomers have identical physical and chemical properties except in two important properties:
- They rotate the plane of polarized light in opposite directions, however in equal amounts. The isomer or enantiomer that rotates the plane counterclockwise or to the left is called the levo isomer and is designated (-). The other isomer rotates the plane clockwise or to the right and is therefore called the dextro isomer and is designated (+). They are also called optical antipodes.
- They react at different rates with other chiral compounds. However, the reaction rates may be so close together that a distinction is not always possible, or they may be so far apart that one isomer reacts much faster than the other or not at all. This explains why many compounds are biologically active while their enantiomers are not. However, enantiomers react at the same rate with achiral compounds.
In addition, the amount of rotation α is not constant for a given enantiomer. The amount of rotation depends on the length of the sample vessel, the temperature, the solvent and concentration (in the case of solutions), the pressure (in the case of gases), and the wavelength of light.
The specific rotation [α] is defined by the formula [α] = α / lc, for solutions, and [α] = α / ld for pure compounds, where α is the observed rotation, l is the length of the cell in decimeters, c is the concentration in grams per milliliter, and d is the density in the same units. The specific rotation is usually reported along with the temperature and wavelength, for examples as [α]25546. [α]D indicated that the rotation was measured with sodium D light at λ= 589 nm. The molecular rotation [M]tλ is the specific rotation times the molecular weight divided by 100.
The reporting structure is important since changes in conditions can change not only the amount of rotation but sometimes also the direction of rotation. For example, one of the enantiomers of aspartic acid, when dissolved in water, has [α]D equal to +4.36° at 20 °C and -1.86° at 90 °C, although the molecular structure is unchanged.
In 1891, Emil Fisher, invented the Fisher projections, a method of how to represent tetrahedral carbons on paper. By this convention, the model is held so that the two bonds in front of the papers are horizontal and those behind the paper are vertical. The ability of these models is limited but they are useful for a quick test if the molecules in question are chiral. In any case, 3D models are much better for the determination of the nature of enantiomers or stereoisomers.
The DL system has been widely used in the past but it is not without faults. Therefore it is only used nowadays for certain groups of molecules, such as carbohydrates and amino acids. The DL system has been replaced by the Cahn-Ingold-Prelog system in which the four groups on an asymmetric carbon are ranked according to a set of sequence rules.
Reference
Any school or college book covering biochemistry and molecular biology including handbooks for amino acids and organic molecule may be reviewed.
http://goldbook.iupac.org/C00772.html
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8.
March’s Advanced Organic Chemistry; Reactions, Mechanisms, and Structure. 6th editions. M.B. Smith and J. March. 2007. Wiley & Sons. Hoboken, NJ.