Antibody drug conjugates- a new way to treat cancer
By Klaus D. Linse
The approval of rituximab, a chimeric monoclonal antibody (mAB) that recognizes the CD20 protein, in 1997 by the U.S. Food and Drug Administration to treat B-cell non-Hodgkin lymphomas resistant to other chemotherapies ushered in an area in which monoclonal antibodies became important components of therapeutic regimens in oncology. To circumvent obstacles encountered with earlier drugs, showing off-target effects such as the targeting of healthy dividing cells as well as other severe side effects, researchers turned to antibody drug conjugates or ADCs to selectively deliver toxic compounds to diseased tissue to be used as “Magic Bullets”. A “Magic Bullet” is a drug or concept first proposed in the early 1900s by PaulEhrlich, a German physician and scientist, that allows the selective targeting of diseased tissue cells. Antibody-drug-conjugates that exhibit improved potency and effectiveness are now considered to be such magic bullets.
The mAB rituximab is used to treat cancers of the white blood system such as leukemias and lymphomas. Monoclonal antibodies now have become a successful class of therapeutic molecules. This success was a result of great progress made in molecular biology and biotechnology which contributed to our understanding of the inner works of the human cell as well of the key players in the immune system. According to the antibody society 35 monoclonal antibodies (mABs) have been approved as therapeutic agents and close to 7 are pending approval (July 2014).
Therapeutic mABs have had a substantial effect on medical care for a wide range of diseases in the past two decades. This is because of the high specificity and ability of mABs to bind target antigens marking these for removal by methods such as complement-dependent cytotoxicity or antibody-dependent cell-mediated cytotoxicity. In addition, mABs can also have therapeutic benefits by binding and inhibiting the function of target antigens. Unfortunately, as medical scientists have found out, antibodies against tumor-specific antigens often lack therapeutic activities.
In recent years it has been found that the conjugation of cytotoxic drugs or radionuclides can expand the utility of mABS. Antibody-drug-conjugates (ADCs) with improved potency and effectiveness are now used as a means to target and deliver a toxic payload to the selected diseased tissue. In recent years this approach has become a major focus for therapeutic research. In the past decade antibodies have been conjugated to a number of cytotoxic drugs using various linker chemistries. Already two such drugs are marketed in the United States. These are ado-trastuzumab emtansine (Kadcyla®) and brentuximab vedotin (Adcetris®), and over 30 ADCs are currently undergoing clinical studies. This makes it likely that more conjugates may be approved in the near future.
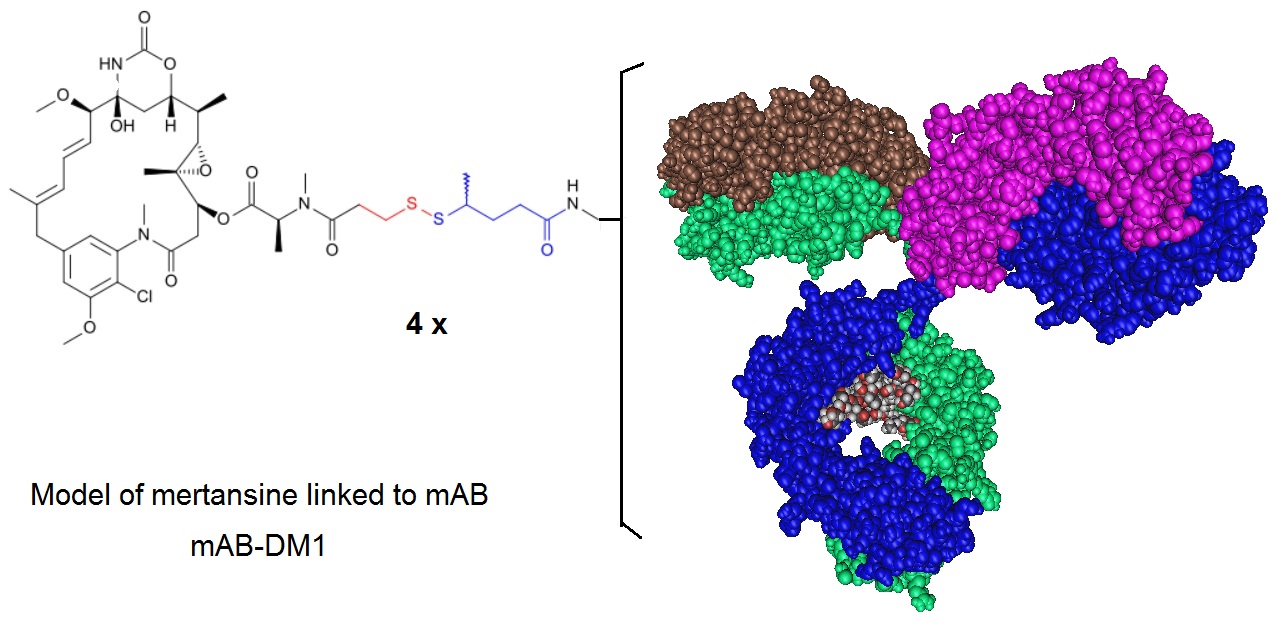
Figure 1: A model of an Antibody Drug Conjugate where a mAb is conjugated to DM1 illustrating the conjugation approach used for Ado-Trastuzumab Emtansine. Ado-trastuzumab emtansine, formerly called Trastuzumab-DM1 (T-DM1), is a HER2 antibody drug conjugate (ADC). In this ADC the trastuzumab antibody is linked to the cell-killing agent, DM1. T-DM1 combines two strategies— anti-HER2 activity and targeted intracellular delivery of the potent anti-microtubule agent, DM1 (a maytansine derivative)—to produce cell cycle arrest and apoptosis. Ado-trastuzumab emtansine is marketed under the brand name Kadcyla and is indicated for use in HER2-positive, metastatic breast cancer patients who have already used taxane and/or trastuzumab for metastatic disease or had their cancer recur within 6 months of adjuvant treatment.
The ADC brentuximag vedotin or Adcetris® combines an anti-CD30 antibody and the drug monomethyl auristatin E (MMAE). It is an anti-neoplastic agent used in the treatment of Hodgkin lymphoma and systemic anaplastic large cell lymphoma and was approved in 2011. In January 2012, the drug label was revised to include a boxed warning of progressive multifocal leukoencephalopathy and death following JC virus infection. The structure of the cAC10-vcMMAE system used for the production of brentuximag vedotin is illustrated in figure 2.

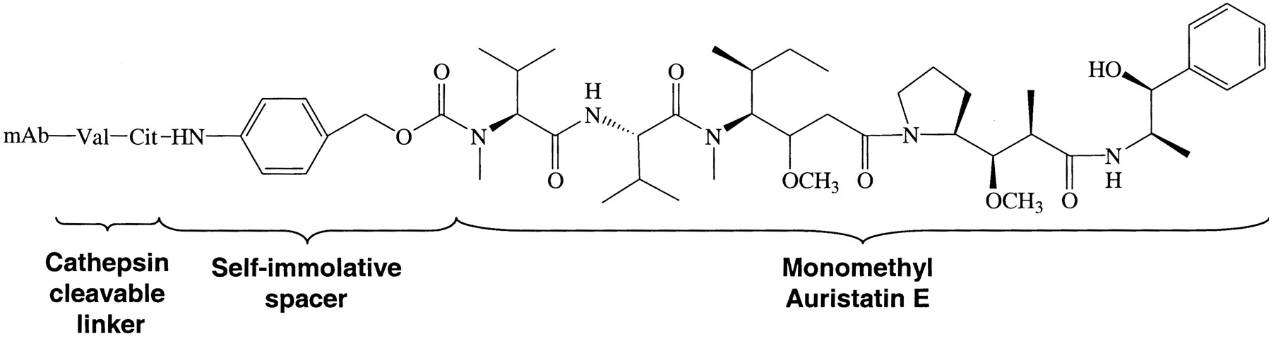
Figure 2: Structure of the cAC10-vcMMAE system used for the production of brentuximag vedotin. Francisco et al. 2003 report how this ADC was prepared. A controlled partial reduction of internal cAC10 disulfides with DTT, followed by addition of the maleimide-vc-linker-MMAE was used. Stable thioether-linked ADCs were formed by the addition of free sulfhydryl groups on the mAbs to the maleimides present on the drugs. cAC10-vcMMAE and the cIgG-vcMMAE used in the report contained approximately 8 drugs/mAb.
The development of ADCs in the past decade has evolved from the use of murine antibodies to conjugate standard chemotherapeutics drugs to fully human antibodies conjugated to highly potent cytotoxic drugs. Critical factors required for the successful development of ADCs, as scientists have learned over the past decade, include target antigen selection, as well as the selection of the best antibody, the correct linker and payload. Typically, ADCs are complex biomolecules composed of an antibody or antibody fragment linked to a biological active cytotoxic or anti-cancerous payload using stable chemical linkers with labile bonds. Other terms used for this type of molecules are the terms “bioconjugate” and “immune-conjugates”. The reasoning for the combination of the unique targeting abilities of antibodies with cancer-killing drugs to create unique ADCs is the potential these drugs can have to treat cancer that are hard to treat with standard classical chemotherapies.
One area of research important for the development and design of ADCs is that of conjugation chemistry. One recent improvement is the implementation of site-specific conjugation methods. Here, the conjugation only occurs at engineered cysteine residues or unnatural amino acids. The result is a homogeneous ADC production and improved ADC pharmacokinetic (PK) properties. The following figure illustrates critical factors that influence the performance of ADC therapeutics.

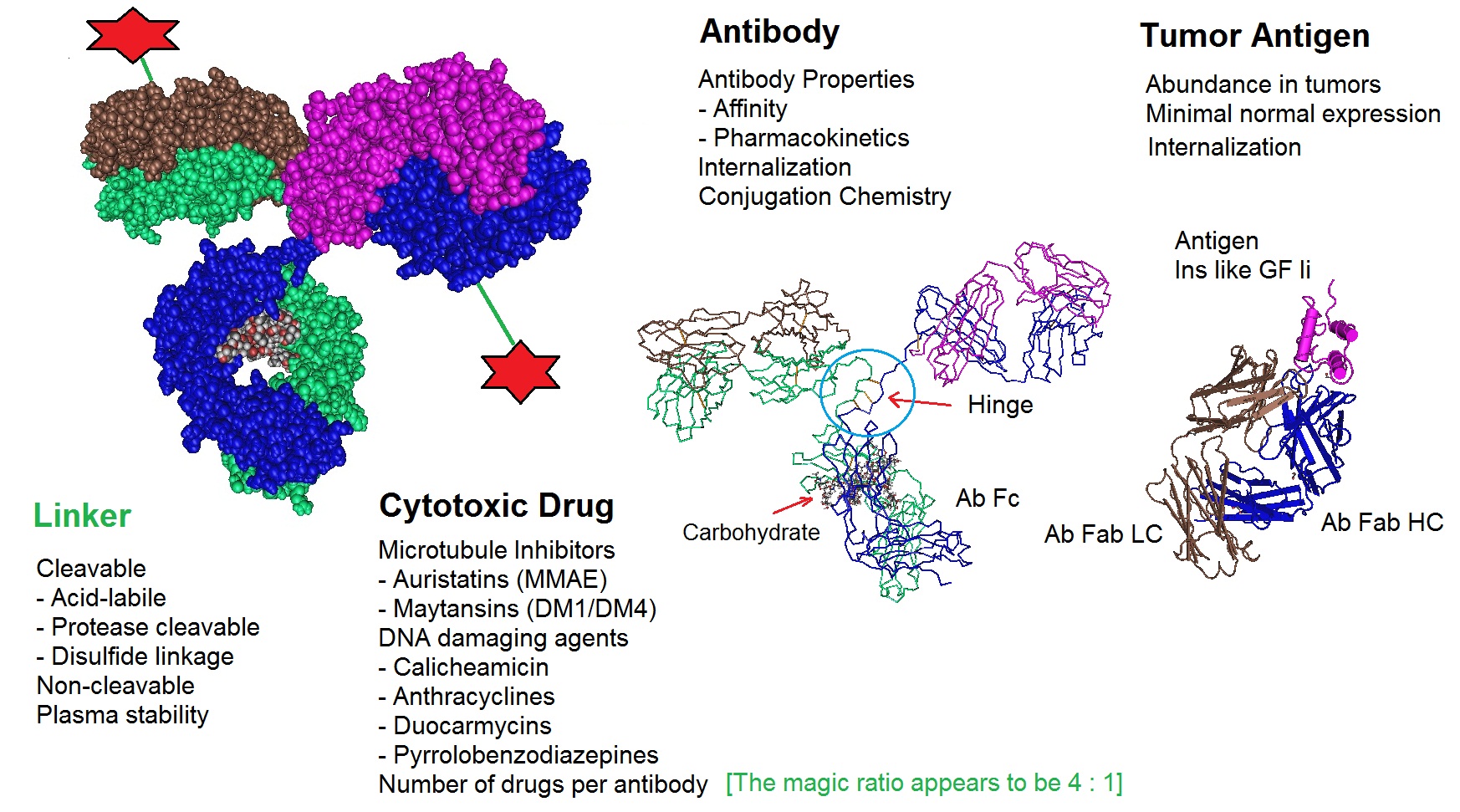
Figure 3: Critical factors that influence Antibody Drug Conjugate or ADC therapeutics. ADCs are designed and produced by conjugating a cytotoxic drug to a monoclonal antibody via a selected linker. All components of an ADC affect the performance of the molecule. The optimization of all molecular parts is essential for the development of successful ADCs.
In the beginning years of ADC development many obstacles had to be overcome. Early ADCs showed little or no therapeutic effect. The primary reason may have been pour target selection. In addition, the use of either chimeric or murine (mouse) antibodies, which can elicit an immunogenic response, and the use of lower potency drugs can also be factors that limit potency. Ultimately, researches learned from their failures and incorporated what they had learned in the next generations of ADCs. Knowledge gained from the development of ADCs has led to a better understanding of the ways in which ADCs function and their clinical performance.
Design of Antibody Drug Conjugates or ADCs
Many papers reporting the use of conjugates have been published since the 1970s. A PubMed search using the terms “Antibody and Drug and Conjugates” retrieved a list of over 2,300 papers covering this subject indicating that this has now become an active research field. Antibody-based therapeutics against cancer are now highly successful in clinical trials and are currently recognized for their potential.
Hundreds of mAbs including bispecific mAbs and multispecific fusion proteins, mAbs conjugated with small molecule drugs and mAbs with optimized pharmacokinetics have already been produced and are in clinical trials. However, many challenges still remain and a deeper understanding of mechanisms for how ADCs work is needed to overcome encountered problems including resistance to therapy, access to molecular targets, as well as to understand the complexity of biological systems and individual variations and how these drug conjugates interact with them.
Unlike conventional chemotherapeutic drugs which do not selectively localize to tumors, antibody-drug conjugates can bind specifically to cells that express the targeted antigen. Therefore these molecules are considered to be ideal vehicles for applications that require delivery of drugs. With the help of various linker strategies antibodies can be conjugated to a variety of drugs or “payloads”.
Ongoing research is investigating new strategies how to conjugate proteins such as IgGs to cytotoxic molecules without altering the natural functions of the proteins.
Antibody or Protein Vehicles
Although whole IgGs already have shown to have many benefits, for certain applications the use of smaller antibody fragments such as monomeric or dimeric fragments, sometimes called diabodies, single-chain variable fragments (scFv) or other small proteins maybe of advantage. This may allow tailoring the delivery time, as indicated by the half-life of the ADC, more precisely. For example, when delivering cytokines to the extracellular space of a tumor, a longer half-life may cause unwanted inflammation.
Linkers
A variety of linkers have now been investigated to connect an antibody or the protein of choice to the drug of choice. The most commonly used linkers are based on amide bond, also called peptide linkers, disulfide bonds or hydrazones as illustrated in the next figure. The selection of the linker determines what happens to the ADC once it is inside a cell. Most linkers are selected so that they can be cleaved by specific mechanisms. Depending on their sequence peptide linkers can be site-specifically cleaved by proteases that recognize the selected peptide sequence motif, or by statistical proteolysis. Disulfide bonds can be broken by the reducing environment of the cytosol and hydrazones can be cleaved by acid-mediated hydrolysis. In addition, ADCs containing proteins such as cytokines maybe produced as fusion proteins.
Payloads
Very potent cytotoxic agents can be used to ensure that the tumor killing can be mediated with acceptably low doses of the ADCs employed. ADCs containing monomethyl auristin E (MMAE) or the maytansioniod DM1 kill tumor cells by inhibiting microtubule polymerization. However, alternative payloads may be used as well. Examples are DNA damaging drugs or cytokines.
The next figure illustrates some building blocks that are used for the design and production of ADCs.

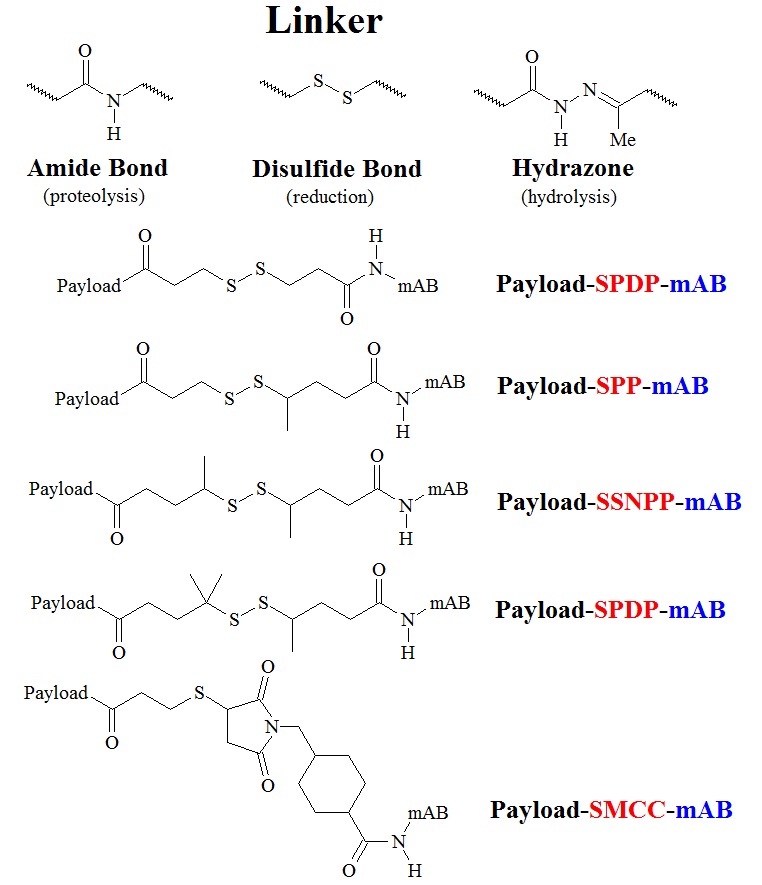
Figure 4: Structures of some building blogs and linkers used in ADC therapeutics. Linkers with an amide bond, a disulfide bond, and a hydrazone bond are illustrated in the upper panel. Structures of drug-SPDP-mAB, drug-SPP-mAB, drug-SSNPP-mAB, and drug-SMCC-mAB are listed in order of stability, least to greatest. The drug-SMCC-mAB conjugate contains a nonreducible linker.
To summarize, ADCs are sophisticated delivery systems for antitumor cytotoxic drugs. Monoclonal antibodies are used to guide the toxin precursor to the target cancer cell. When the target is reached the prodrug can be converted chemically or enzymatically to the parent drug and unfold its activity to kill the cancer. On its journey from the blood vessels to the molecular target in the tumor tissue the ADC is exposed to different conditions.
-
During circulation: The ADC must behave like a naked antibody when circulating in the plasma. The linkers used must be stable in the blood stream. The goal is to limit the damage to healthy tissue since decomposition or decay would relase the cytotoxin before being delivered to the target.
-
Antigen Binding: The conjugated mAB needs to retain high immunoaffinity thus the attachment of the cytotoxic drug must not disturb the binding specificity.
-
Internalization: For the ADC to work well, a sufficient intracellular concentration of the drug must be achieved. This maybe one of the biggest challenges since antigen targets on cell sufaces are often present in limited numbers. In addition, the internalization process for antigen-antibody complexes is often inefficient.
-
Drug Release: Once inside the cell, the ADC needs to efficiently release the selected cytotoxic drug in its active form inside the tumor cell.
-
Drug Action: The potency of the selected drug must be sufficient to kill the tumor cells, often even at low concentration. To achieve this the use of very potent drugs is necessary. Compounds that are too toxic when tested as a stand-alone chemotherapy appear to be quite suitable candidates to be used as ADC payloads.
The insight gained during the development of ADCs in the past decade have resulted in a number of new strategies and drugs. This is indicated by the flood of publications in this research field.
Selected References
ADC poster: http://www.nature.com/nbt/extra/adc/index.html
Franco Dosio, Paola Brusa and Luigi Cattel; Immunotoxins and Anticancer Drug Conjugate Assemblies: The Role of the Linkage between Components. Toxins 2011, 3, 848-883; doi:10.3390/toxins3070848
Ducry L, Stump B.; Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 2010 Jan;21(1):5-13. doi: 10.1021/bc9002019.
Joseph A. Francisco, Charles G. Cerveny, Damon L. Meyer, Bruce J. Mixan, Kerry Klussman, Dana F. Chace, Starr X. Rejniak, Kristine A. Gordon, Ron DeBlanc, Brian E. Toki, Che-Leung Law, Svetlana O. Doronina, Clay B. Siegall, Peter D. Senter, and Alan F. Wahl; cAC10-vcMMAE, an anti-CD30–monomethyl auristatin E conjugate with potent and selective antitumor activity. August 15, 2003; Blood: 102 (4).
SEAN L KITSON, DEREK J QUINN, THOMAS S MOODY, DAVID SPEED, WILLIAM WATTERS, DAVID ROZZELL; Antibody-Drug Conjugates (ADCs) – Biotherapeutic bullets. Chemistry Today - vol. 31(4) July/August 2013.
Nilvebrant J, Åstrand M, Georgieva-Kotseva M, Björnmalm M, Löfblom J, Hober, S.; (2014) Engineering of Bispecific Affinity Proteins with High Affinity for ERBB2 and Adaptable Binding to Albumin. PLoS ONE 9(8): e103094. doi:10.1371/journal.pone.0103094.
Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blättler WA, Lambert JM, Chari RV, Lutz RJ, Wong WL, Jacobson FS, Koeppen H, Schwall RH, Kenkare-Mitra SR, Spencer SD, Sliwkowski MX; Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008 Nov 15;68(22):9280-90. doi: 10.1158/0008-5472.CAN-08-1776.
Panowksi S, Bhakta S, Raab H, Polakis P, Junutula JR.; Site-specific antibody drug conjugates for cancer therapy. MAbs. 2014 Jan-Feb;6(1):34-45. doi: 10.4161/mabs.27022.
Sassoon I, Blanc V.; Antibody-drug conjugate (ADC) clinical pipeline: a review. Methods Mol Biol. 2013;1045:1-27. doi: 10.1007/978-1-62703-541-5_1.
Feng Tiana, Yingchun Lua, Anthony Manibusana, Aaron Sellersa, Hon Trana, Ying Suna, Trung Phuonga, Richard Barnetta, Brad Hehlia, Frank Songa, Michael J. DeGuzmanb, Semsi Ensarib, Jason K. Pinkstaffc, Lorraine M. Sullivanc, Sandra L. Birocc, Ho Chod, Peter G. Schultze, John DiJoseph, Maureen Dougher, Dangshe Ma, Russell Dushing, Mauricio Lealh, Lioudmila Tchistiakovai, Eric Feyfanti, Hans-Peter Gerber, and Puja Sapra; A general approach to site-specific antibody drug conjugates. (2014) PNAS 111, 5, 1766-1771.