Crotamine is a cell-penetrating peptide that accumulates in the nucleus and can be used to transport DNA into replicating cells.
In addition, it is thought that crotamine has the potential to transport drugs into mammalian cells without the need for specific receptors.
Crotamine is a highly basic polypeptide of 42 amino acids that has an isoelectric point (pI) of 10.3 and a molecular mass of 4.8 kDa. Crotamine is found in the venom of the Brazilian rattlesnake Crotalus durissus terrificus. Crotamine was first isolated in 1947 from the venom of this snake. Crotamine selectively inhibits and interferes with the functioning of Kv1.3 channels and promotes the permeability of bacterial membranes. Furthermore, crotamine has been reported recently to possess both antitumoral and antibacterial activities.
Amino Acid Sequence
ykqchkkgghcfpkekiclppssdfgkmdcrwrwkcckkgsg
Molecular weight
4890.01 g/mol (reduced), 4884.01 (oxidized).
Amino Acid Composition
C = 6 (14.29), D = 2 ( 4.76), E = 1 ( 2.38), F = 2 ( 4.76), G = 5 (11.90), H = 2 ( 4.76), I = 1 ( 2.38), K = 9 (21.43), L = 1 ( 2.38), M = 1 ( 2.38), P = 3 ( 7.14), Q = 1 ( 2.38), R = 2 ( 4.76), S = 3 ( 7.14), W = 2 ( 4.76), Y = 1 ( 2.38).
Crotamine contains three disulfide bridges and several isoforms have been characterized. The overall peptide fold is homologous to antimicrobial peptides (AMPs) belonging to the α-defensin, β-defensin and insect defensin families. Crotamine has the same structural scaffold as mammalian α-defensins and β-defensins, a three-stranded β-sheet core and a framework of loops stabilized by six disulfide-linked cysteines.
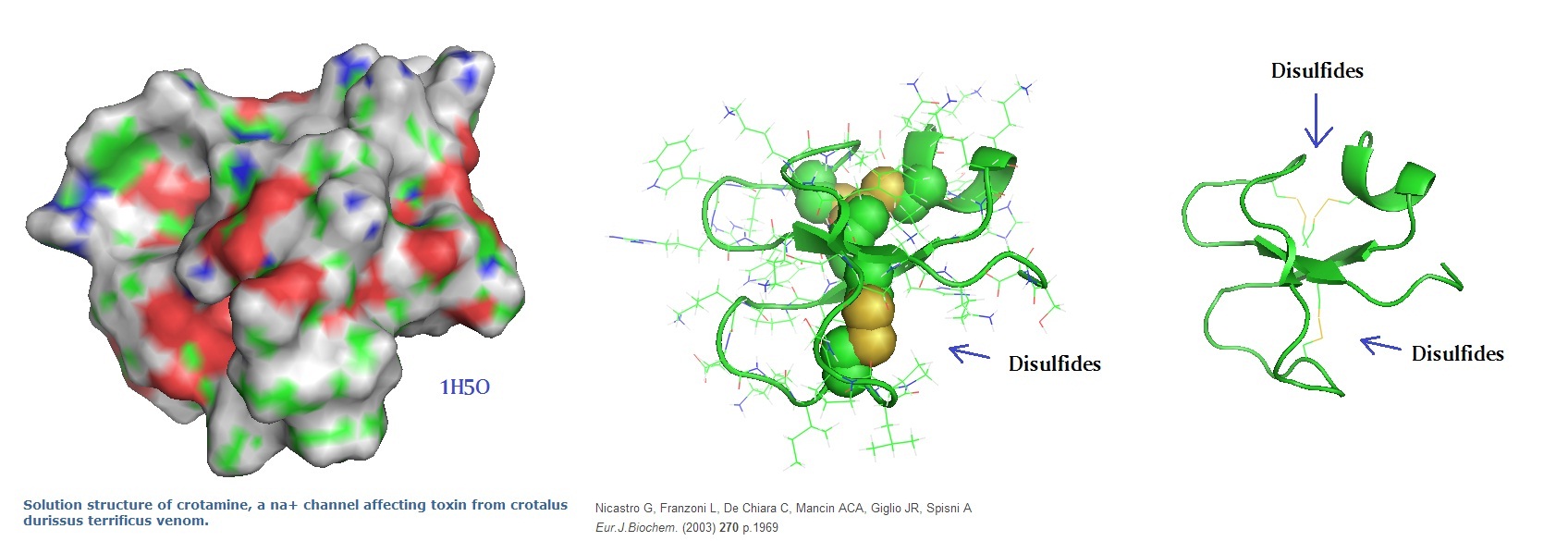
Figure 1: Molecular models of the cell-penetrating polypeptide Crotamine from the venom ofCrotalus durissus terrificus as reported by Nicastro et al in 2003.
Nicastro G, Franzoni L, de Chiara C, Mancin AC, Giglio JR, Spisni A.; Solution structure of crotamine, a Na+ channel affecting toxin from Crotalus durissus terrificus venom. Eur J Biochem. 2003 May;270(9):1969-79.
“Abstract
Crotamine is a component of the venom of the snake Crotalus durissus terrificus and it belongs to the myotoxin protein family. It is a 42 amino acid toxin cross-linked by three disulfide bridges and characterized by a mild toxicity (LD50 = 820 micro g per 25 g body weight, i.p. injection) when compared to other members of the same family. Nonetheless, it possesses a wide spectrum of biological functions. In fact, besides being able to specifically modify voltage-sensitive Na+ channel, it has been suggested to exhibit analgesic activity and to be myonecrotic. Here we report its solution structure determined by proton NMR spectroscopy. The secondary structure comprises a short N-terminal alpha-helix and a small antiparallel triple-stranded beta-sheet arranged in an alphabeta1beta2beta3 topology never found among toxins active on ion channels. Interestingly, some scorpion toxins characterized by a biological activity on Na+ channels similar to the one reported for crotamine, exhibit an alpha/beta fold, though with a beta1alphabeta2beta3 topology. In addition, as the antibacterial beta-defensins, crotamine interacts with lipid membranes. A comparison of crotamine with human beta-defensins shows a similar fold and a comparable net positive potential surface. To the best of our knowledge, this is the first report on the structure of a toxin from snake venom active on Na+ channel. PMID: 12709056 [PubMed - indexed for MEDLINE]”
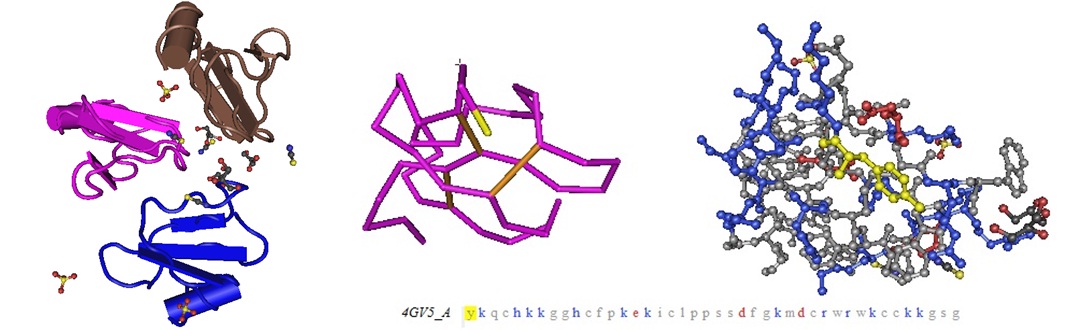
Figure 2: Molecular models of the cell-penetrating polypeptide Crotamine from the venom ofCrotalus durissus terrificus as reported by Coronado et al in 2013.
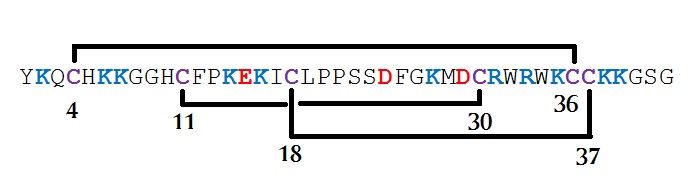
Figure 3: Disulfide bond connectivity of crotamine.
Coronado MA, Gabdulkhakov A, Georgieva D, Sankaran B, Murakami MT, Arni RK, Betzel C.; Structure of the polypeptide crotamine from the Brazilian rattlesnake Crotalus durissus terrificus. Acta Crystallogr D Biol Crystallogr. 2013 Oct;69(Pt 10):1958-64. doi: 10.1107/S0907444913018003. Epub 2013 Sep 20.
“Abstract
The crystal structure of the myotoxic, cell-penetrating, basic polypeptide crotamine isolated from the venom of Crotalus durissus terrificus has been determined by single-wavelength anomalous dispersion techniques and refined at 1.7 Å resolution. The structure reveals distinct cationic and hydrophobic surface regions that are located on opposite sides of the molecule. This surface-charge distribution indicates its possible mode of interaction with negatively charged phospholipids and other molecular targets to account for its diverse pharmacological activities. Although the sequence identity between crotamine and human β-defensins is low, the three-dimensional structures of these functionally related peptides are similar. Since crotamine is a leading member of a large family of myotoxic peptides, its structure will provide a basis for the design of novel cell-penetrating molecules.”