Photocleavable oligonucleotides have many applications in biological and medicinal research. Photoactive groups can function as protecting groups of oligonucleotides and for a variety of other bioactive or reporter molecules. Molecules functionalized with a photoactive group are also known as caged compounds. Photocleavable or photolabile groups have been used heavily in synthetic organic chemistry for a variety of applications. For example, molecular releasing systems utilize photochemical reactions based on energy transfer between a quencher or sensitizer and the photoactive group. Even molecular logic gates or networks can be designed using multiple components in combination with photocleavable groups or linker molecules.
The o-nitrobenzyl derivatives have gained enormous popularity in synthetic chemistry and as building blocks for oligonucleotide and peptide synthesis.
The function of the photolabile group is based on the photoisomerization of an o-nitrobenzyl alcohol derivative into a corresponding o-nitrobenzaldehyde upon irradiation with UV light and the release of a free carboxylic acid (Zhao et al. 2012: o-nitrobenzyl alcohol derivatives).
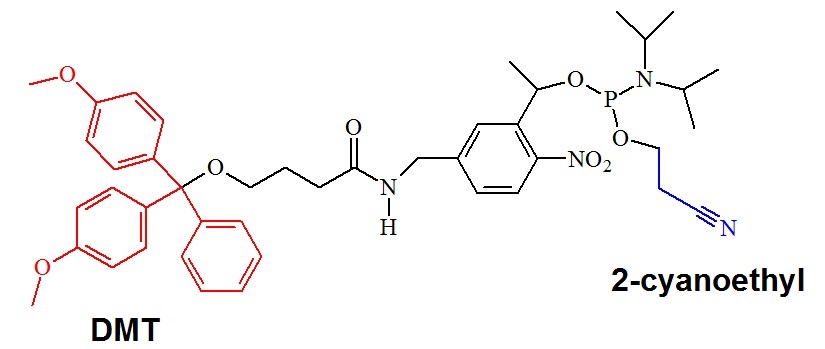
Figure 1: Structure of the PC-Spacer Phosphoramidite [4-(4,4'-Dimethoxytrityloxy) butyramidomethyl)-1-(2-nitrophenyl)-ethyl]- 2-cyanoethyl-(N,N-diisopropyl)-phosphoramidite].
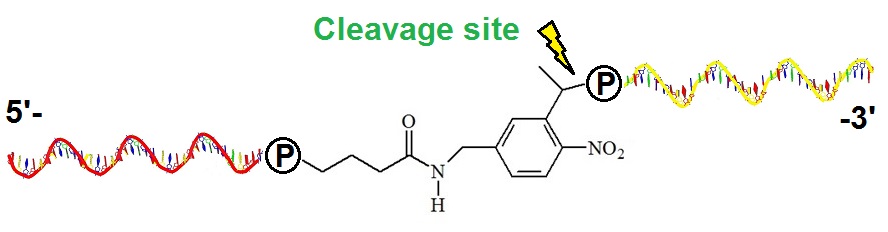
Figure 2: Example of oligonucleotide attachment points to the PC spacer moiety. This non-nucleosidic photocleavable spacer molecule can be incorporated into oligonucleotides either on the 5 ‘- or 3’-end. If only incorporated on the 5’-end oligonucleotides can be immobilized on glass slides or other surfaces. This spacer type is used in a variety of applications, for example, for the synthesis of photocleavable block copolymers. To test if the photocleavage is quantitative a fluorophore can be attached at one end such that the dye is released in a way that allows monitoring of the reaction. For example, from 5’- tagged oligonucleotides immobilized to a surface on the 3’-end.
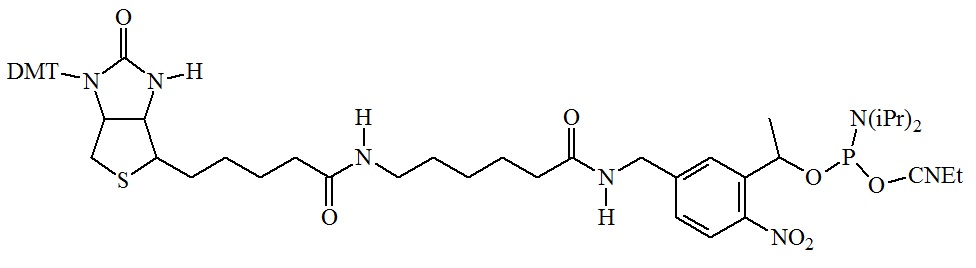
Figure 3: A photocleavable biotin spacer useful for phosphoramidite oligonucleotide synthesis. This spacer allows the capture of modified oligonucleotides using the streptavidin system and the rapid, quantitative removal of the biotin spacer using near UV light at 300 to 350 nm. The released oligonucleotides are now suitable for other biological applications such as gene construction as part of a cloning experiment.
Applications of 5’- Photocleavable Oligonucleotides:
Affinity isolation and purification of nucleic acids binding proteins
Cassette mutagenesis.
Diagnostic assays requiring release of the probe-target complex or specific markers.
DNA-programmed single oxygen production.
DNAzyme deactivation and reactivation.
Multiple non-radioactive probes for DNA/RNA blots.
Nucleic acid sensing.
Nucleobase-Caging.
Optochemical control of cellular processes.
Optochemical control of DNA:Protein interactions.
PCR.
Photoactivated molecular beacons for drug release.
Photosensitized Singlet Oxygen Production.
Spatially-addressable photorelease of probe-target complexes or marker molecules for diagnostics.
Templated catalysis.
Examples from the literature:
Olejnik and others in 1998 reported the synthesis of oligonucleotides containing a photocleavable amino group conjugated to the 5'-terminal phosphate of oligonucleotides. This 5' photocleavable amino group allows the introduction of various amine-reactive molecules to synthetic oligonucleotides as well as their immobilization on activated solid supports. Irradiation with near-UV light selectively cleaves off the photocleavable bond on the 5'-phosphate. Photocleavable conjugates containing biotin, digoxigenin and fluorophores such as tetramethylrhodamine can be prepared as well.
Clo and others in 2006 demonstrated an on-and-off switching 1O2 sensitizer controlled by DNA sequences. The photosensitizer pyropheophorbide-a (P) is attached or conjugated to a short 15-mer nucleotide sequence and a “black hole quencher 3” (Q) is attached to a 21-mer oligonucleotide which complements the 15-mer oligonucleotide. P and Q are brought into close proximity after DNA assembly by hybridization. The singled state of P is quenched by Q via FRET or by contact-mediated electron exchange.
Zhou et al. in 2012 described a mass spectrometry-based assay for the analysis of protein kinase activities in a multiplexing format. This assay can be used for screening inhibitors against multiple kinases in parallel useful for drug discovery and predictive diagnostics.
For this assay to work, oligonucleotides are tagged or conjugated to peptides. The peptides selected are substrates for kinases allowing efficient capture from solution-phase kinase reactions by annealing to the complementary sequence tethered to PEG-passivated superparamagnetic microparticles.
Synthesis of peptide-oligonucleotide conjugates with bifunctional photocleavable cross-linkers enables a reversible conjugation. The relative state of phosphorylation is detected using mass spectrometry after washing away contaminants and photoreleasing the peptides.
Reference
Emiliano Cló, John W. Snyder, Niels V. Voigt, Peter R. Ogilby, and, and Kurt V. Gothelf; DNA-Programmed Control of Photosensitized Singlet Oxygen Production. Journal of the American Chemical Society 2006 128 (13), 4200-4201. DOI: 10.1021/ja058713a. https://pubs.acs.org/doi/abs/10.1021/ja058713a.
Olejnik, J., Krzymanska-Olejnik, E., & Rothschild, K. J. (1998). Photocleavable aminotag phosphoramidites for 5’-termini DNA/RNA labeling. Nucleic Acids Research, 26(15), 3572–3576. https://academic.oup.com/nar/article/26/15/3572/2360254.
Zhou, G., Khan, F., Dai, Q., Sylvester, J. E., & Kron, S. J. (2012). Photocleavable Peptide-Oligonucleotide Conjugates for Protein Kinase Assays by MALDI-TOF MS. Molecular bioSystems, 8(9), 2395–2404. http://doi.org/10.1039/c2mb25163a.
---...---