A gapmer oligonucleotide modified with bridged nucleic acids (BNAs) used as an antisense oligonucleotide (ASO) for the reduction of circulating LDL-cholesterol levels lowers the level of hepatic PCSK9.
In Hypercholesterolemia, high levels of cholesterol are present in the blood. In humans with this condition, during a blood test, elevated levels of cholesterol and lipoproteins are detected. The presence of high plasma cholesterol levels, together with normal plasma triglyceride levels, causes the rise of cholesterol and apolipoprotein B (apoB)-rich lipoproteins, called low-density lipoprotein (LDL) that characterize Hypercholesterolemia.
Cholesterol, a fatty molecule, occurs naturally in the human body since it is essential in cell walls. Cholesterol is also the primary molecule for the biosynthesis of hormones. The intestine absorbs eaten fat and cholesterol transports them to the liver through the blood. Two main types of cholesterol exist in blood: low-density lipoprotein (LDL) cholesterol (the “bad” cholesterol) and high-density lipoprotein (HDL) cholesterol (the “good” cholesterol).
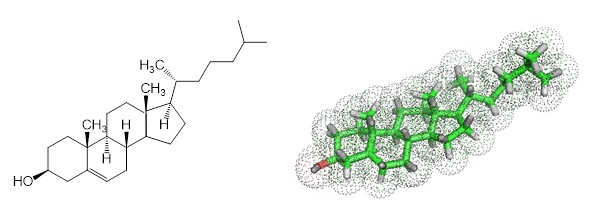
Figure 1: Chemical structure and model of cholesterol.
The low-density lipoprotein (LDL) receptor binds and internalizes cholesterol-containing particles.
Since cholesterol is insoluble in water and in body fluids, it must be transported by a water-soluble carrier. The LDL particle is a sphere 20 to 25 nm in diameter with an outer surface that is a monolayer membrane made up of phospholipids and cholesterol in which one molecule of the large protein called apo-B is embedded. In the nonpolar core, cholesterol is esterified through the single hydroxyl group to a long-chain fatty acid. After transport into the cell by a process called endocytosis, LDL particles are transported to lysosomes. In lysosomes, the apo-B protein is degraded to amino acids and the cholesterol esters are hydrolyzed to cholesterol and fatty acids. The cell incorporates cholesterol directly into the cell membranes or re-esterifies it and stores it as lipid droplets.
Familial Hypercholesterolemia is characterized by very high levels of cholesterol in the blood. This condition is inherited. The body of people that have familial Hypercholesterolemia is unable to excrete extra cholesterol, and therefore it builds up in the blood. Humans with Familial Hypercholesterolemia are at a high risk of developing a form of heart disease called coronary artery disease at a young age.
The proprotein convertase subtilisin/kexin type 9 (PCSK9) protein controls the number of low-density lipoprotein receptors. PCSK9 is a regulatory factor in Familial Hypercholesterolemia (FH). Hence it is an attractive therapeutic target for the reduction of low-density lipoprotein (LDL) cholesterol. PCSK9 is responsible for the degradation of hepatic low-density lipoprotein receptor (LDLR). Low levels of hepatic PCSK9 activities are associated with reduced levels of circulating LDL-cholesterol.
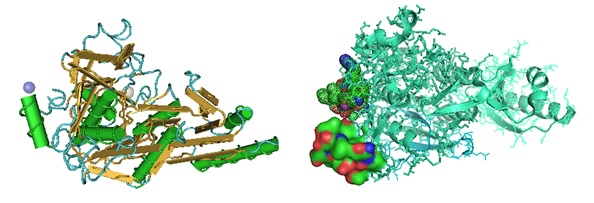
Figure 2: Structure of Proprotein Convertase Subtilisin/kexin Type 9 protein in complex with 2 peptides. [PDB 5VLK].
In 2020, Gupta et al. reported the use of a gapmer oligonucleotide modified with bridged nucleic acids (BNAs) as an antisense oligonucleotide (ASO) for the reduction of circulating LDL-cholesterol levels by lowering the level of hepatic PCSK9. Gupta et al. designed an LNA antisense oligonucleotide (LNA ASO) complementary to the human and mouse PCSK9 mRNA. A 13-nucleotide long gapmer (Sequence: GTctgtggaaGCG; uppercase BNA/LNA, lowercase DNA) with phosphorothioate internucleoside linkages was used. Gupta et al. used Human hepatocytes derived cell lines HepG2 and HuH7 and a pancreatic mouse β-TC3 cell line known to express high endogenous levels of PCSK9 for the study. The study showed that the ASO efficiently reduced the mRNA and protein levels of PCSK9. Also, after transfection, levels of LDL-receptor (LDLR) increased. In vivo efficacy of the ASO was further investigated in mice by tail vein intravenous administration of BNA ASO in saline solution. The level of PCSK9 mRNA was reduced by ∼60 %, lasting more than 16 days. Hepatic LDLR protein levels were significantly up-regulated by 2.5 to 3 folds for at least eight days and ∼two-fold for 16 days. Analysis of liver alanine aminotransferase (ALT) levels showed that the cells tolorated long term LNA ASO treatment. Also, the treatment did not cause hepatotoxicity.
A second study investigated two bridged nucleic acids (BNA/LNA) antisense oligonucleotides targeting PCSK9 for a lowering of low-density lipoprotein cholesterol levels in nonhuman primates (Lindholm et al. 2012). The study showed that antisense bridged nucleic acid gapmers reduced PCSK9 messenger RNA and serum PCSK9 protein by 85%, resulting in a 50% reduction in circulating low-density lipoprotein cholesterol. Also, the treatment reduced serum total cholesterol levels to the same extent as circulating low-density lipoprotein cholesterol with no change in high-density lipoprotein levels.
Gapmers used for the study were complementary to human (accession #NM174936) and Macaca fascicularis (cynomolgus monkey) PCSK9 mRNA with the sequences: TGCtacaaaacCCA and GTctgtggaaGCG.
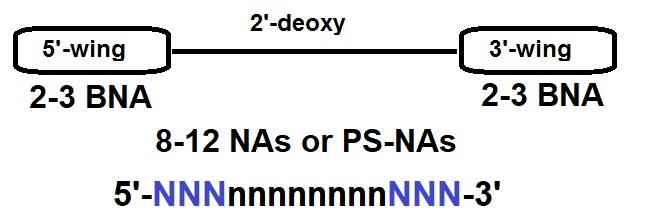
Figure 3: Gapmer Design Example. A gapmer is a chimeric antisense oligonucleotide that contains a central block of deoxynucleotide monomers sufficiently long to induce RNase H cleavage. The central block of a gapmer is flanked by blocks of 2’-O modified ribonucleotides or other artificially modified ribonucleotide monomers such as bridged nucleic acids (BNAs). In a gapmer these modified nucleic acids protect the internal block from nuclease degradation. Natural unmodified DNA, as well as modified DNA analogs such as phosphorothiote DNA analogs can be used to stabilize RNA molecules useful as gapmers for therapeutic approaches.
Reference
Gupta N, Fisker N, Asselin MC, Lindholm M, Rosenbohm C, Ørum H, Elmén J, Seidah NG, Straarup EM (2010). Deb S (ed.). "A locked nucleic acid antisense oligonucleotide (LNA) silences PCSK9 and enhances LDLR expression in vitro and in vivo". PLoS ONE. 5 (5): e10682. Bibcode:2010PLoSO...510682G. doi:10.1371/journal.pone.0010682. [PMC]
Lindholm MW, Elmén J, Fisker N, Hansen HF, Persson R, Møller MR, Rosenbohm C, Ørum H, Straarup EM, Koch T. PCSK9 LNA antisense oligonucleotides induce sustained reduction of LDL cholesterol in nonhuman primates. Mol Ther. 2012 Feb;20(2):376-81. doi: 10.1038/mt.2011.260. Epub 2011 Nov 22. PMID: 22108858; PMCID: PMC3277239. [PMC]
Lodish, Baltimore, Berk, Darnel, Matsudaira, Zipursky; Molecular Cell Biology. Scientific American Books. 1995.
Zhang Y, Ultsch M, Skelton NJ, Burdick DJ, Beresini MH, Li W, Kong-Beltran M, Peterson A, Quinn J, Chiu C, Wu Y, Shia S, Moran P, Di Lello P, Eigenbrot C, Kirchhofer D.; Discovery of a cryptic peptide-binding site on PCSK9 and design of antagonists. Nat Struct Mol Biol. 2017 Oct;24(10):848-856. doi: 10.1038/nsmb.3453. Epub 2017 Aug 21. [PDB]
---...---