Gapmers are short deoxyribonucleic acid-based antisense oligonucleotides. Antisense oligonucleotides are chemically modified to enhance the pharmacological properties of RNase H1-antisense oligonucleotides (ASOs). Common modifications of gapmer antisense oligonucleotides include a phosphorothioate (PS) backbone and different 2’-modifications. Usually, each end of an ASO gapmer contains 2’-modifications. The PS backbone is a common component of therapeutic nucleic acids.
Generally, gapmers are designed to hybridize or bind to target ribonucleic acid (RNA) sequences to silence a gene through induced cleavage of the targeted sequence by RNase H.
Intracellular proteins are known to bind antisense oligonucleotides (ASOs) containing phosphorothioate bonds. PS-ASOs are known to bind to plasma proteins, including albumin and α2-macroglobulin, that prevent ASOs from being rapidly discarded through urinary excretion. Compared to PO-ASOs, PS-ASOs enhance cellular uptake both in vitro and in vivo.
Therapeutic PS-ASOs enter cells mainly through endocytic pathways and are released from endocytic particles into the cytosol and nucleus to bind to complementary RNAs via base-pairing. 2′-modifications also affect ASO activity, often by increasing the binding affinity of ASOs to RNA targets.
Liang et al. have identified and characterized more than 50 intracellular proteins interacting with ASOs. However, the mechanism of how ASOs interact with these proteins remains largely unknown.

MALAT PS MOE DNA gamer ASO in double stranded form (PDB ID 7ZVN).
2’-MOE PS nucleotides are shown in cyan. PS DNA gapmer in black. All cytidines in the ASO are methylated (5meC). Location of mismatches are highlighted with red dots.
In 2016, Liang et al. studied the protein-binding effects of ASOs containing phosphorothioate (PS) in the backbone and 2′-modifications in the ribose. The researchers utilized affinity selection and competition assays to show that the PS backbone modification dominates protein binding. The study found that compared to a PO-backbone, PS-modified ASOs bind more proteins more tightly. Also, the number of PS-modified nucleotides affects the binding of many proteins to ASOs. ASOs containing less than 10 PS-modified nucleotides interacted with proteins significantly weaker than ASOs with greater PS numbers, and the 2′-modifications in the wings of gapmer ASOs affected protein binding as well.
According to Liang et al., the Hsp90 protein interacts more strongly with PS-ASOs containing locked-nucleic acid (LNA) or constrained-ethyl-bicyclic-nucleic acid ((S)-cEt) modifications than with 2′-O-methoxyethyl (MOE). Also, ASOs bind to the mid-domain of the Hsp90 protein. ASOs containing the hydrophobic 2′-modifications (S)-cEt or LNA in the 5′-wing interact with Hsp90.
Reduction of the Hsp90 protein decreased the activity of PS-ASOs with 5′-LNA or 5′-cEt wings but not with 5′-MOE wings. This study by Liang et al. indicates that the Hsp90 protein enhances the activity of ASOs modified with PS and LNA moieties or PS and (S)-cEt moieties, suggesting that different chemical modifications can improve the therapeutic efficacy of PS-ASOs.
In 2020, Hyjek-Składanowska reported a crystal structure-based model of the DNA-binding domain of the PC4 protein in complex with a PS 2′-OMe DNA gapmer. In the structure, each PC4 dimer contains two DNA-binding interfaces. The 5′-terminal 2′-OMe PS flank is bound by one interface. The regular PS DNA central part is bound in opposite polarity as a hairpin-like structure by the other interface. Binding to the ASO induces the formation of a dimer of dimers of PC4. The base pairing between regions of the ASO stabilizes the binding to each PC4 dimer. The PS nucleic acid interacts with the protein through electrostatic and hydrophobic contacts. NanoBRET binding assay confirmed these contacts.
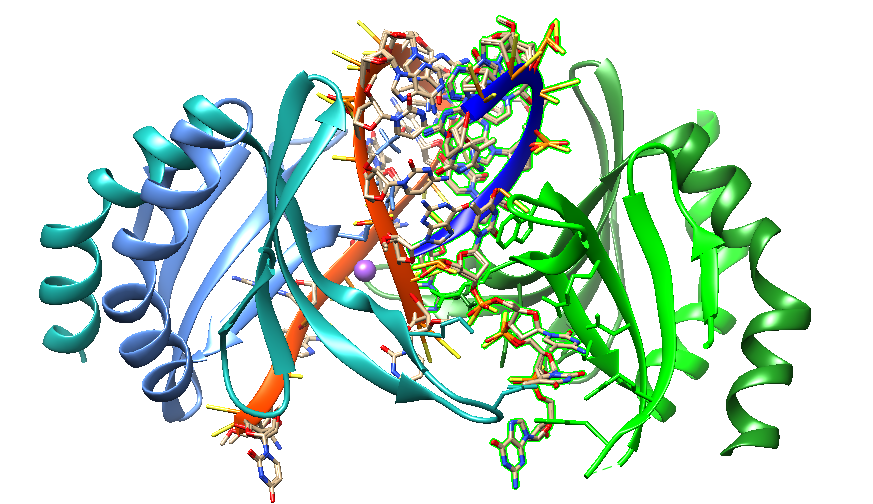
Human Transcription Cofactor PC4 DNA-binding domain in complex with full phosphorothioate 5-10-5 2'-O-methyl DNA gapmer antisense oligonucleotide [6YCS].
Hyjek-Składanowska et al. suggest that this structure provides insights into the molecular forces governing the interactions of PS ASOs with cellular proteins. Furthermore, it provides a potential model for how these interactions can cause cellular toxicity. Pandey et al. (2021) state that the interaction between human positive coactivator 4 (PC4) and the tumor suppressor protein p53 is crucial in initiating apoptosis. PC4 is an abundant nuclear protein. Since PC4 assisted-p53-dependent apoptosis may play a central role in certain neurodegenerative diseases, disrupting p53-PC4 interactions may be a promising drug target for specific disease pathologies.
More recently, in 2023, Hyjek-Składanowska et al. reported the crystal structure of human Annexin A2 in complex with a phosphorothioate 5-10 2”-MOE DNA gapmer ASO. The research group co-crystallized the C-terminal domain (CTD) core of annexin A2 (AnxA2) with a phosphorothioate ( PS) 2′-methoxyethyl (MOE) DNA gapmer ASO. The crystal structure revealed that unique hydrophobic interactions between PS groups, lysine and arginine residues account for the enhanced affinity of PS ASOs to the protein surface. These results demonstrate that this interaction mechanism appears to be a general phenomenon observed not only for nucleic acid-binding proteins but may also explain interactions of ASOs proteins.
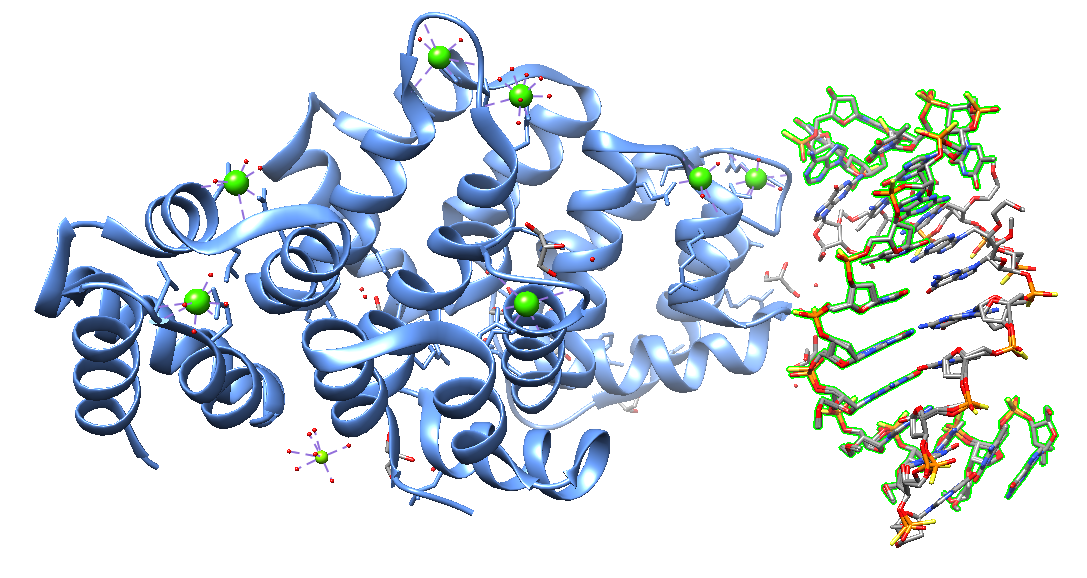
Crystal structure of human Annexin A2 in complex with full phosphorothioate 5-10 2'-methoxyethyl DNA gapmer antisense oligonucleotide solved at 1.87 Å resolution [7ZVN].
The annexin A (ANXA) protein family is a well-known tissue-specific multigene family encoding calcium (Ca2+) phospholipid-binding proteins. ANXA proteins play essential roles in cancer progression, proliferation, invasion, and metastasis in several diseases.
Reference
Crooke ST, Vickers TA, Liang XH. Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res. 2020 Jun 4;48(10):5235-5253. [PMC]
Hyjek-Składanowska M, Vickers TA, Napiórkowska A, Anderson BA, Tanowitz M, Crooke ST, Liang XH, Seth PP, Nowotny M. Origins of the Increased Affinity of Phosphorothioate-Modified Therapeutic Nucleic Acids for Proteins. J Am Chem Soc. 2020 Apr 22;142(16):7456-7468. [PubMed][pdb/6ycs]
Hyjek-Składanowska M, Anderson BA, Mykhaylyk V, Orr C, Wagner A, Poznański JT, Skowronek K, Seth P, Nowotny M. Structures of annexin A2-PS DNA complexes show dominance of hydrophobic interactions in phosphorothioate binding. Nucleic Acids Res. 2023 Feb 22;51(3):1409-1423. [PMC]
Liang XH, Shen W, Sun H, Kinberger GA, Prakash TP, Nichols JG, Crooke ST. Hsp90 protein interacts with phosphorothioate oligonucleotides containing hydrophobic 2'-modifications and enhances antisense activity. Nucleic Acids Res. 2016 May 5;44(8):3892-907. [PMC]
Pandey B, Dev A, Chakravorty D, Bhandare VV, Polley S, Roy S, Basu G. Insights on the disruption of the complex between human positive coactivator 4 and p53 by small molecules. Biochem Biophys Res Commun. 2021 Nov 12;578:15-20. [PubMed]
Zhang H, Zhang Z, Guo T, Chen G, Liu G, Song Q, Li G, Xu F, Dong X, Yang F, Cao C, Zhong D, Li S, Li Y, Wang M, Li B, Yang L. Annexin A protein family: Focusing on the occurrence, progression and treatment of cancer. Front Cell Dev Biol. 2023 Mar 3;11:1141331. [PMC]
---...---
" Bio-Synthesis provides a full spectrum of high quality custom oligonucleotide modification services including 5'-triphosphate and back-bone modifications, conjugation to fatty acids, biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides".
---...---