Pyrene and its derivatives are polycyclic aromatic hydrocarbons acting as fluorophores. Pyrene-based molecular probes display monomeric fluorescence emission peaks between 375 to 405 nm. The absorption peak for pyrene is 360 nm, and the typical emission peak is 387 nm.
Pyrene exhibits an additional excimer band at ~460 nm when two pyrene fluorophores are in spatial proximity. Excimer-forming pyrene pairs have a long Stokes shift, the difference between absorption and emission maxima of around > 100 nm, a long exciting lifetime, a temperature-independent emission, and good chemical stability. The pyrene excimer forms when two pyrene molecules are in proximity as a short-lived dimer in an electronically excited state. The pyrene excimer has a long fluorescence lifetime of >40 nanoseconds allowing the selective detection of the excimer using time-resolved emission spectra. The fluorescence from pyrene can be selectively detected after the fluorescence from the cellular background has decayed (Marti et al. 2006). The spectral features of pyrene allows biochemist the study of conformation and conformational changes in biomolecules, for example, in proteins (Bains et al. 2011).
Pyrene probes are sensitive to their microenvironment. Pyrene exhibits several monomer fluorescence emission peaks that report on the polarity of the probes microenvironment. The appearance of an additional band at longer wavelengths reflects the presence of another pyrene molecule in spatial proximity (~10 Å).
Because of their strong fluorescent emission in living cells, pyrene-based fluorescent probes are widely used as fluorescent probes. Their low cytotoxicity, high fluorescence quantum yield, easy modification, and good cell permeability make them the molecules of choice for the design of specific cellular probes. One example is the spatial imaging of biomolecules in cells, including various RNA molecules.
The modification of biomolecules with pyrene allows the design of specific molecular probes for the detection and study of metal cations, toxic metal cations, the sensing of anions, small neutral molecules, different pH, and fluorescence-based bioimaging in living cells.
Yamana et al. showed in 1999 that pyrene-modified oligonucleotides bind to their complementary DNA and RNA in aqueous solutions. Oligonucleotides with a 2’-pyrene-modified ribonucleoside have different fluorescence properties when bound to DNA or RNA targets than in the unbound stage.
Time-resolved emission spectra (TRES) using pyrene probes enabled Marti et al. the selective detection of mRNAs in the presence of cellular extracts. Pyrene-based probes allow imaging of mRNAs in cellular environments with background fluorescence, for example, by localizing neuronal mRNA. Optimized hybridization probes for detecting messenger RNA sequences enable their selective detection.
Excimer probes contain two oligonucleotide strands labeled with a pyrene molecule. The excitation of the free monomeric probes in solution at 350 nm yields a defined spectrum with a maximum of 390 and 410 nm. Excimer complexes are only weekly associated in the electronic ground state but interact strongly when electronically exited. As shown by Hoche et al., a characteristic red-shifted fluorescence identifies excimers with a lifetime of several ns. In fluorescence excitation spectra of pyrene and its dimer, the S1( 1B2u) ← S0( 1A1g) transition of the monomer is observed at 367.4 nm; however, the strongly vibronically coupled S2 state is observed at 321.5 nm, and the dimer (Py)2 exhibits a broad and unstructured electronic spectrum around 370 nm. Hoche et al. studied the mechanism of the pyrene excimer formation to achieve a detailed picture of the excimer formation dynamics. They showed that dynamical effects could be essential in designing novel organic functional materials such as pyrene-labeled oligonucleotides or peptides.
|
.jpg)
Pyrene is a colorless solid. Both, the solid and solution have a slight blue fluorescence.
|
|
|
Figure 1: Pyrene
|
Figure 2: Binary pyrene probes in the absence of target sequence (above) and after hybridization with the target sequence (below). [Marti et al.]
|
In 2002 Smirnov et al. showed that DNA polymerases could specifically insert a hydrophobic pyrene deoxynucleotide opposite a tetrahydrofuran. The solutions structure of the duplex solved via NMR indicated that complementary and stacking interactions are sufficient to maintain the stability of DNA structures, including replicons. Figure 3 illustrates models of a 13mer duplex containing a pyrene-tetrahydrofuran (P-F) pair.
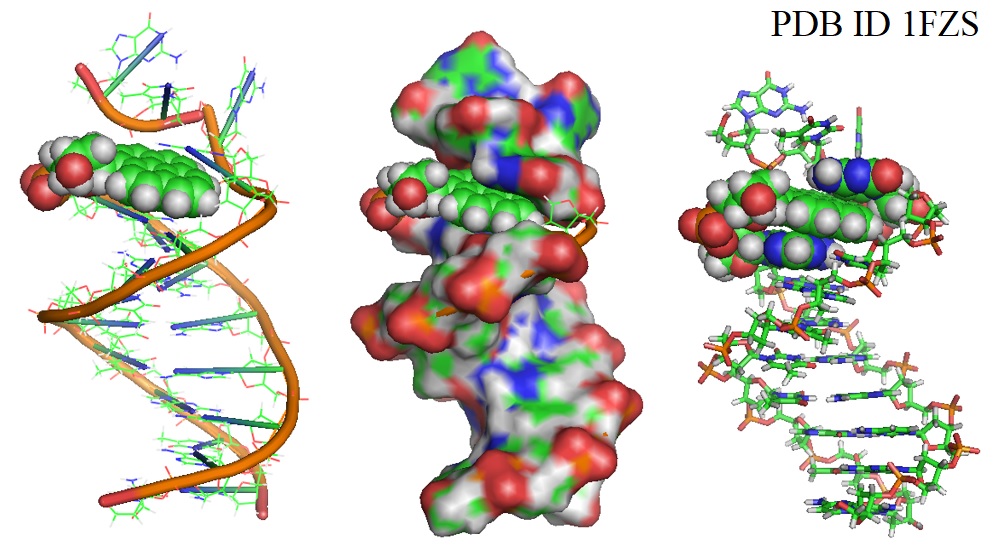
Figure 3: DNA duplex with pyrene paired at abasic site (PDB ID 1FZS).
The model revealed that the pyrene ring stays inside the helix, close to its baseless partner in the duplex. The researchers suggested that the stability of a P-F pair is due to its ability to mimic the Watson-Crick structure. DNA polymerases preferentially incorporated pyrene opposite abasic sites but failed to extend primer DNA containing a P·F pair at the template/primer junction. Smirnov et al. suggested that this occurs because of the absence of a hydrogen bond acceptor in the minor groove of the crescent duplex known to facilitate primer extension.
Reference
1FZS DNA WITH PYRENE PAIRED AT ABASIC SITE
Bains G, Patel AB, Narayanaswami V. Pyrene: a probe to study protein conformation and conformational changes. Molecules. 2011 Sep 14;16(9):7909-35. [PMC]
Bains GK, Kim SH, Sorin EJ, Narayanaswami V. The extent of pyrene excimer fluorescence emission is a reflector of distance and flexibility: analysis of the segment linking the LDL receptor-binding and tetramerization domains of apolipoprotein E3. Biochemistry. 2012 Aug 7;51(31):6207-19. [PMC]
Hoche, J., Schmitt, H.C., Humeniuk, A., Fischer, I., Mitrića, R., and Röhr ,M. I. S.; The mechanism of excimer formation: an experimental and theoretical study on the pyrene dimer. Physical Chemistry Chemical Physics 19(36) 2017, 25002-25015. [RSC]
Martí AA, Li X, Jockusch S, Li Z, Raveendra B, Kalachikov S, Russo JJ, Morozova I, Puthanveettil SV, Ju J, Turro NJ. Pyrene binary probes for unambiguous detection of mRNA using time-resolved fluorescence spectroscopy. Nucleic Acids Res. 2006 Jun 12;34(10):3161-8. [PMC]
Smirnov S, Matray TJ, Kool ET, de los Santos C. Integrity of duplex structures without hydrogen bonding: DNA with pyrene paired at abasic sites. Nucleic Acids Res. 2002 Dec 15;30(24):5561-9. [PMC]
Yamana K, Iwase R, Furutani S, Tsuchida H, Zako H, Yamaoka T, Murakami A. 2'-Pyrene modified oligonucleotide provides a highly sensitive fluorescent probe of RNA. Nucleic Acids Res. 1999 Jun 1;27(11):2387-92. [PMC]
--...---
Bio-Synthesis provides a full spectrum of oligonucleotide and peptide synthesis including bio-conjugation services as well as high quality custom oligonucleotide modification services, back-bone modifications, conjugation to fatty acids and lipids, cholesterol, tocopherol, peptides as well as biotinylation by direct solid-phase chemical synthesis or enzyme-assisted approaches to obtain artificially modified oligonucleotides, such as BNA antisense oligonucleotides, mRNAs or siRNAs, containing a natural or modified backbone, as well as base, sugar and internucleotide linkages.
The synthesis of FRET-oligonucleotides or peptides is also possible.
Bio-Synthesis also provides biotinylated mRNA and long circular oligonucleotides.
---...---