Fluorescence‐based peptide assays allow screening of protease inhibitor compounds in a high throughput format. Fluorescence resonance energy transfer (FRET) peptides are useful tools for the study of protease and peptidase specificities. FRET peptides enable continuous monitoring of cleavage reactions as well as the determination of enzyme activities.
In general, FRET peptides allow investigation of any biochemical reaction causing a change in the physical distance between donor and acceptor molecule.
Typical applications for FRET peptides are
(i) the functional characterization of peptidases, proteases, kinases, or phosphatases,
(ii) kinetic characterization of peptidases, proteases, kinases, or phosphatases,
(iii) screening and detection of new proteolytic enzymes, or
(iv) conformational investigation of peptide folding.
Hydrolysis of a peptide bond between a donor-acceptor pair generates fluorescence. FRET peptides allow enzyme activity studies at nanomolar concentrations. A FRET event is the transfer of energy from an initially excited donor molecule, a dye (Dye 1), to an acceptor molecule, also a dye (Dye 2). FRET is a distant dependent dipole-dipole interaction without the emission of a photon. A fluorescent donor group attached to one of the amino acid residues of the peptide transfers energy to a quenching acceptor group attached to another residue in the sequence. Theodor Foerster explained the mechanism of this energy migration or transfer in 1948. When the emission spectrum of the fluorophore overlaps with the absorption spectrum of the acceptor, FRET occurs.
Efficient FRET requires
(i) the proximity between donor and acceptor, usually in the range of 10 to 100 Ångström,
(ii) the overlap of the absorption spectrum of acceptor with the emission spectrum of the donor, and
(iii) transition dipole orientation of donor and acceptor to be approximately parallel.
Energy transfer in FRET can happen in two ways
(i) conversion of energy transfer into molecular vibration (in the case of dark quencher groups), or
(ii) emission of transferred energy as light of longer wavelength (in the case is the acceptor molecule is fluorescent).
The physical separation of the two dyes generates a flurescent signal. Flurescent signals differ for different dye pairs.
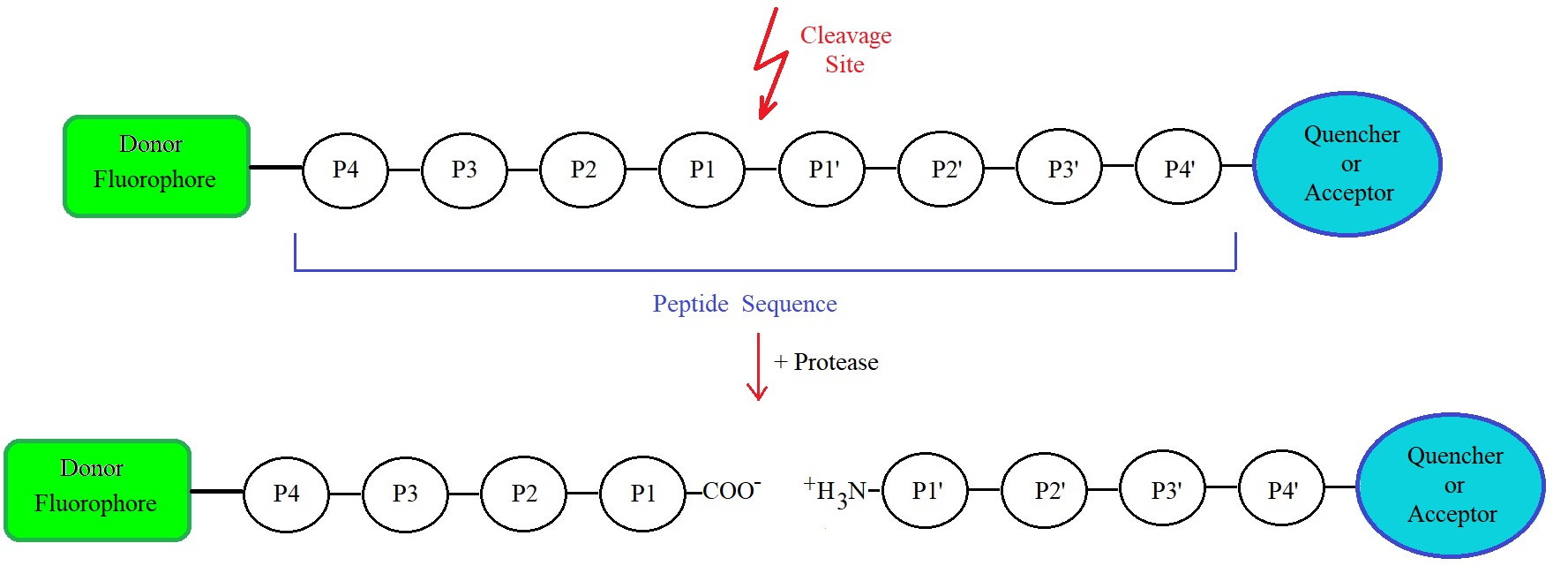
Figure 1: Schematic representation of a FRET peptide and FRET mechanism. Upon cleavage of any peptide bond within the amino acid sequence, fluorescence occurs. P1, P2, P3, and P4 are designated for amino acid residues in the N-terminal direction from the cleavage site or cleaved peptide bond. P1’, P2’, P3’, and P4’ designate amino acids in the C-terminal direction. The terminology used is the one, according to Schechter & Berger (1967).
Typically, FRET peptides use ortho-aminobenzoic acid (Abz) or 5-((2-aminoethyl)amino)naphthalene-1-sulfonic acid (EDANS) as the fluorescent group and 2,4-dinitrophenyl (DNP), 4-dimethylaminobenzene-4’-sulfonyl chloride (DAPSYL) or N-(2,4-dinitrophenyl)ethylenediamine (EDDnP) as the quencher. Many more dye and quencher pairs are now commercially available.
Proteolytic enzymes are fundamental in regulating biological processes and are often associated with pathological conditions. Viruses, such as coronaviruses, including SARS-CoV-2 (COVID-19), utilize proteases for their viral life cycle.
The 3C-like (3CL) protease is essential for the life cycle of acute severe respiratory syndrome-coronavirus (SARS-CoV). This protease is a key target for antiviral drugs or agents. Hsu et al., in 2004, reported virus inhibitory effects for compounds phenylmercuric acetate, hexachlorophene, and thimerosal, when studying virus-infected Vero E6 cells. Phenylmercuric acetate and thimerosal are widely used as antimicrobial preservatives in parenteral and topical pharmaceutical formulations. Phenylmercuric acetate is used in cosmetics, as an antimicrobial preservative, as a bactericide in parenterals, and eye‐drops, and as a spermicide. Hexachlorophene is commonly found in soaps and scrubs and is known as a cholinesterase inhibitor. The metals Hg2+, Zn2+, and Cu2+ as well as 1‐hydroxypyridine‐2‐thione zinc, and zinc salts such as zincum gluconicum (Zenullose) also showed inhibitory effects in the study. Zinc is also known as an effective treatment for the common cold.
Coronaviruses belong to a family of positive-stranded RNA viruses containing one of the largest viral RNA genomes. The SARS coronavirus replicase gene produces two overlapping translation products, polyproteins 1a (∼450 kDa) and 1ab (∼750 kDa). Polyprotein sequences are highly conserved within other coronaviruses.
The internally encoded 3C-like proteinase processes polyproteins 1a and 1ab by cleaving at specific sites. The processing event releases functional proteins necessary for virus replication.
The SARS 3C-like proteinase is highly conserved among all the released SARS coronavirus genomes and also homologous to other coronavirus 3C-like proteinases. Fan et al. reported 11 cleavage sites of the 3C-like proteinase on the SARS polyprotein, as revealed by sequence analysis. SARS 3C-like proteinase cleaves 11 peptides containing all the 11 cleavage sites in the viral polyprotein sequence with different efficiency. SARS 3C-like proteinase tolerates Phe, Val, and Met residues at P2 position. However, mainly P1, P2, and P1′ positions determine the specificity of the protease.
For the study of substrate specificity of SARS 3C-like proteinase, Fan et al. cloned, expressed, and purified the protein. The 11 peptides covering the 11 cleavage sites on the virus polyprotein were studied.
The cloned and purified SARS 3C-like proteinase cleaved the substrate peptides containing the polyprotein cleavage sites. FRET peptides using these sequences allow the study of coronavirus SARS 3C-like proteinases.
Table 1: Coronavirus Cleavage Site Peptides with focus on SARS-CoV-2
|
Polyprotein 1ab
|
SEQUENCE
|
AAs
|
Origin
|
|
Orf1ab
|
|
|
|
|
CS 1: 3257 to 3271
|
Itsavlq/sgfrkmAF
|
15
|
SARS-CoV-2
|
|
CS 1: 3258 to 3268
|
TSAVLQ/SGFRK
|
11
|
SARS-CoV-2
|
|
CS 1: 2897 to 2911
|
Svnstlq/sglrkmAQ
|
15
|
FIPV
|
|
CS 1: 3266 to 3281
|
mfgvnlq/sgkttsmf
|
15
|
hCoV-229E
|
|
CS 1: 3385 to 3403
|
vstsflq/sgivkmVS
|
15
|
CoV HKU1; ABD75543.1
|
|
CS 2: 3560 to 3574
|
QCSGVTFQ/SAVKRTI
|
15
|
SARS-CoV-2
|
|
CS 2: 3541 to 3551
|
SGVTFQ/GKFKK
|
11
|
SARS CoV TW11
|
|
CS 3: 3854 to 3864
|
KVATVQ/SKMSD
|
11
|
SARS-CoV-2
|
|
CS 4: 3937 to 3947
|
NRATLQ/AIASE
|
11
|
SARS-CoV-2
|
|
CS 5: 4135 to 4145
|
SAVKLQ/NNELS
|
11
|
SARS-CoV-2
|
|
CS 6: 4248 to 4258
|
ATVRLQ/AGNAT
|
11
|
SARS-CoV-2
|
|
CS 7: 4364 to 4374
|
REPLMQ/SADAS
|
11
|
SARS-CoV; ACZ72150.1
|
|
CS 7: 4387 to 4396
|
REPMLQ/SADAQ
|
11
|
SARS-CoV-2
|
|
CS 7: 4389 to 4403
|
PMLQ/SADAQ/SFLNRV
|
15
|
SARS-CoV-2
|
|
CS 8: 5319 to 5329
|
PHTVLQ/AVGAC
|
11
|
SARS-CoV-2
|
|
CS 9: 5920 to 5930
|
NVATLQ/AENVT
|
11
|
SARS-CoV-2
|
|
CS 10: 6447 to 6457
|
TFTRLQ/SLENV
|
11
|
SARS-CoV-2
|
|
CS 11: 6793 to 6803
|
FYPKLQ/SSQAW
|
11
|
SARS-CoV-2
|
|
CS 11: 6770 to 6780
|
FYPKLQ/ASQAW
|
11
|
SARS-CoV
|
CS = cleavage site. PP1ab FIPV = polyprotein 1ab: SARS [Feline infectious peritonitis virus, FIPV]
The relative cleavage efficiencies of the SARS 3C-like proteinase for the 11 peptides representing the eleven cleavage sites in SARS coronavirus polyprotein as determined by Fan et al. (2004) are illustrated in Figure 2. The SARS 3C-like proteinase used for the studied was a cloned and purified SARS-CoV 3C-like proteinase. A peptide cleavage assay using synthetic peptides containing the cleavage sites in combination with HPLC allowed determination of the relative enzyme activity.
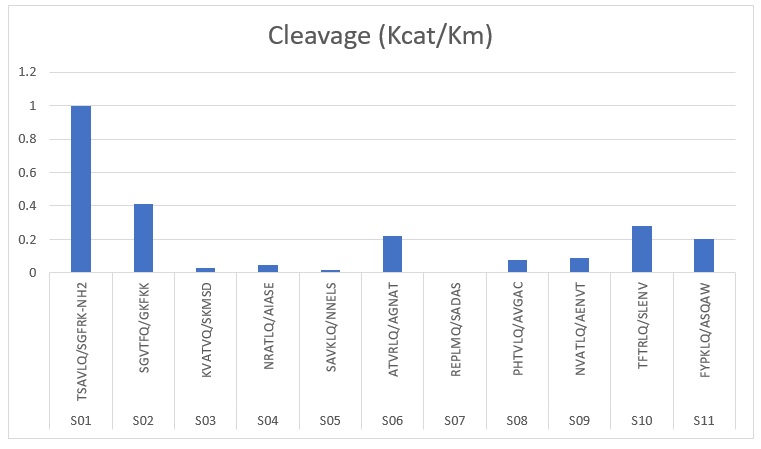
Figure 2: Relative cleavage efficiencies for eleven peptides (Source: Fan et al. 2004).
FRET oligonucleotides are also available for biochemical studies. Similar dye pairs enable the design of FRET oligonucleotides.
Reference
Amos CARMEL and Arieh YARON; An Intramolecularly Quenched Fluorescent Tripeptide as a Fluorogenic Substrate of Angiotensin-I-Converting Enzyme and of Bacterial Dipeptidyl Carboxypeptidase. Eur. J. Biochem. 87, 265-273 (1978). [PubMed]
Carmona Adriana K., Juliano Maria Aparecida, Juliano Luiz. The use of Fluorescence Resonance Energy Transfer (FRET) peptidesfor measurement of clinically important proteolytic enzymes. An. Acad. Bras. Ciênc. 2009 Sep; 81( 3 ): 381-392. [PubMed]
Dye and quencher pairs: https://www.biosyn.com/faq/dye-and-quencher-pair.aspx#!
Fan K., Wei P., Feng Q., Chen S., Huang C., Ma L., Lai B., Pei J., Liu Y., Chen J., Lai L. Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase. J. Biol. Chem. 2004;279:1637–1642. [PubMed]
Förster, Th.; 1948. Intermolecular energy migration and fluorescence. Ann. Phys. 2: 55-75. [PubMed]
Hsu JT, Kuo CJ, Hsieh HP, Wang YC, Huang KK, Lin CP, Huang PF, Chen X, Liang PH. Evaluation of metal-conjugated compounds as inhibitors of 3CL protease of SARS-CoV. FEBS Lett. 2004 Sep 10;574(1-3):116-20. [PMC]
Kuo CJ, Chi YH, Hsu JT, Liang PH. Characterization of SARS main protease and inhibitor assay using a fluorogenic substrate. Biochem Biophys Res Commun. 2004 Jun 11;318(4):862-867. [PMC]
Schechter I, Berger A.; On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157-62. [PubMed]
Sekar RB, Periasamy A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J Cell Biol. 2003 Mar 3;160(5):629-33. [PMC]
Bio-Synthesis Inc. is pleased to offer a large variety of bioconjugates, oligonucleotides and peptides, modified and unmodified, for a number of research applications, including COVID 19 testing, analysis and vaccine development!
---...---