To overcome disadvantages of drugs with poor water solubility different strategies such as the conjugation of the drug to small molecules, carbohydrates, oligonucleotides, peptides, or other molecules have been developed by many research groups from all over the world in recent decades.
The solubility of the drug Paclitaxel in aqueous media can be increase by conjugating Paclitaxel to aptamers, oligonucleotides, or DNA linked gold nanoparticles.
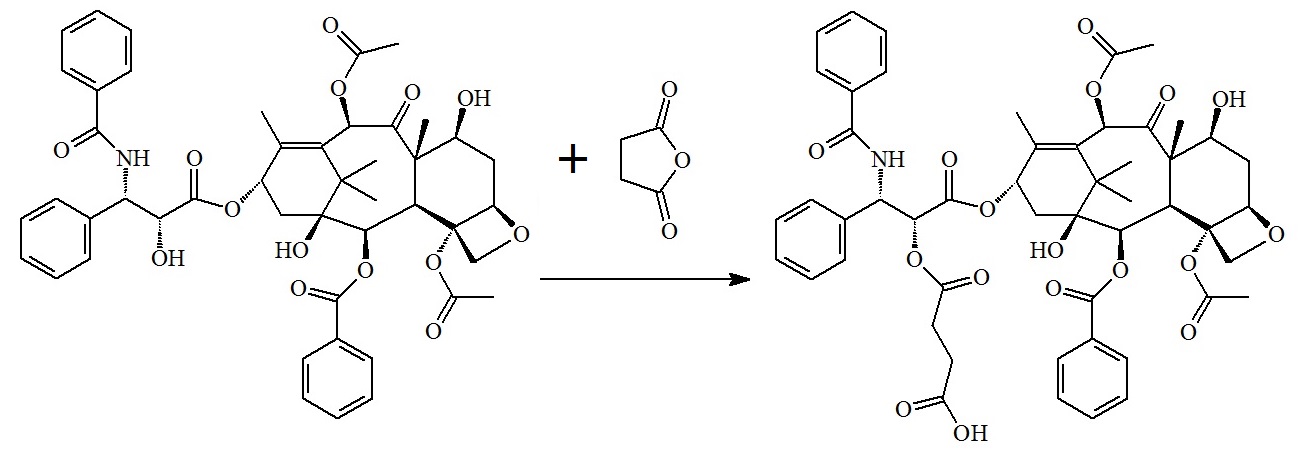
A good example is the conjugation of functionalized Paclitaxel to gold nanoparticles via a DNA linker. In this approach Zhang et al. used succinic anhydride to add a functional extender group to the drug that now allows conjugation to oligonucleotides containing an amino group on one of it's terminal ends followed by conjugating this conjugate to nano-gold particles. First, Paclitaxel is reacted with succinic anhydride to generated Paclitaxel carboxylic acid which now allows conjugation to an amino group on a DNA oligonucleotide.
The following steps were needed for the production of fluorescent Paclitaxel-DNA-gold nanoparticles.
(1) Synthesize propyl thiol-(3’-end) DNA oligomers with a terminal amino group
(5’-end) needed for covalent attachment of Paclitaxel.
(2) Modify Paclitaxel with succinic anhydride to create Paclitaxel carboxylic acid.
(3) Covalently attach Paclitaxel carboxylic acid to the modified oligonucleotide
using EDC/Sulf-NHS.
(4) Purify and QC the derivative (PTX-DNA) using MALDI-MS.
(5) Immobilize the derivative on citrate-stabilized gold nanoparticles (AuNPs).
(6) Remove excess PTX-DNA.
(7) Resuspend PTX-DNA-AuNPs.
For the synthesis of the Paclitaxel-fluorescein-DNA conjugate (Zhang et al. 2011) succinic anhydride was used to modify Paclitaxel by the reaction with succinic anhydride to create Paclitaxel carboxylic acid.
A modified DNA oligonucleotide containing a amino group at the 5’-end, an internal fluorophore, and a thiol group at the 3'-end was covalently attached to Paclitaxel carboxylic acid to yield the Paclitaxel-DNA (PTX-DNA) compound. (Structural models are not to scale).
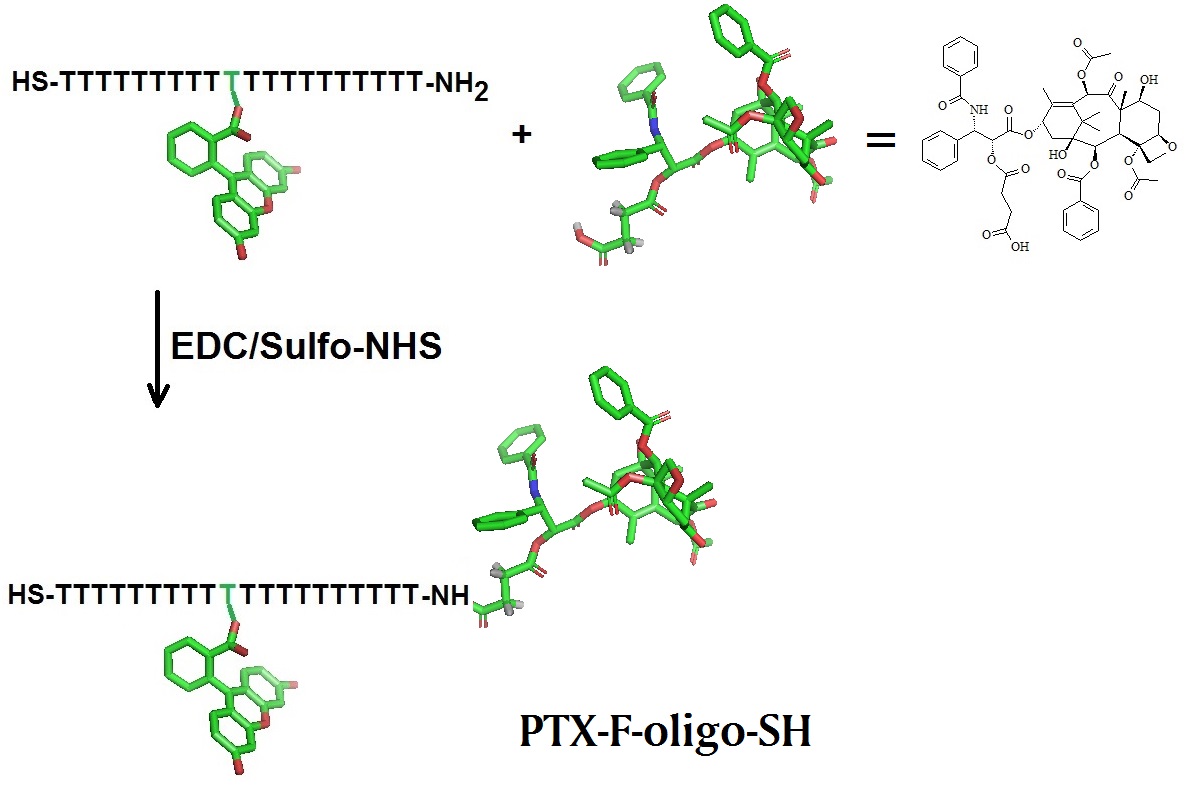
Immobilization of PTX-DNA on citrate-stabilized gold nanoparticles (AuNPs) yielded the Paclitaxel-DNA gold nanoparticle conjugate (PTX-DNA@AuNPs). HPLC is used for the purification of intermediate products and MALDI-TOF-MS is used for QA/QC.
Zhang et al. showed that covalently attached hydrophobic Paclitaxel onto gold nanoparticles via a DNA linker resulted in enhanced hydrophilicity and stability of the conjugate. Cellular internalization, delivery, and distribution of the drug conjugate was monitored in human breast adenocarcinoma cells and uterine sarcoma cells with the help of confocal fluorescence microscopy. Furthermore, the cell-killing activity of paclitaxel was enhanced in vitro against several cancer cell lines when delivered as the gold nanoparticle conjugate.
The authors of the referenced papers suggested that this approch and other similar approaches can be used for other biological useful compounds such as peptides, small interfering RNA (siRNA), gadolinium complexes, antibodies, as well as aptamers.
To conclude, these types of conjugates represent a powerful biochemical platform for combinatorial therapy, bioimaging, and biodiagnostics.
Reference
Li, Fangfei; Lu, Jun; Liu, Jin; Liang, Chao; Wang, Maolin; Luyao; Li, Defang; Yao, Houzong; Zhang, Qiulong; Wen, Jia; Zhang, Zong-Kang; Li, Jie; Lv, Quanxia; He, Xiaojuan; Guo, Baosheng; Guan, Daogang; Yu, Yuanyuan; Dang, Lei; Wu, Xiaohao; Li, Yongshu; Chen, Guofen; Jiang, Feng; Sun, Shiguo; Zhang, Bao-Ting; Lu, Aiping; Zhang, Ge (2017). A water-soluble nucleolin aptamer-paclitaxel conjugate for tumor-specific targeting in ovarian cancer. Nature Communications 1390, 8, 1.
Zhang, X.-Q., Xu, X., Lam, R., Giljohann, D., Ho, D., & Mirkin, C. A. (2011). A Strategy for Increasing Drug Solubility and Efficacy through Covalent Attachment to Polyvalent DNA-Nanoparticle Conjugates. ACS Nano, 5(9), 6962–6970. http://doi.org/10.1021/nn201446c.
---...---