The utility of 6-thio-2’-deoxyguanosine for novel telomerase-dependent anti-cancer therapy
Unlike prokaryotic genomes consisting of circular DNA, the eukaryotic genomes consist of linear DNA, which is organized into distinct chromosomes. The DNA winds around the basic protein histone to form nucleosomes, which undergo compaction to form a highly condensed chromatin. Chromosomes are organized into distinct structural components, ex. centromere that mediates chromosome segregation during mitosis (Alberts, 2015). Telomeres at the ends of chromosomes are comprised of numerous repeats of simple DNA sequence such as TTAGGG in the case of humans.
The main function of telomere is to protect chromosome ends from degradation and avoid genetic instability arising from the fusion with other chromosomes at the termini. To prevent DNA repair/recombination from occurring at the chromosome ends, the DNA sequence repeats and a single stranded 3’-overhang of telomere fold back on itself to form a loop (T loop) with the single strand invading the duplex to anneal with one of the two strands (D loop) (Xu et al., 2016). This ‘capping’ function of telomere is mediated by Shelterin proteins, which incudes TRF1, TRF2, POT1, RAP1 and others.
In most organisms, the length of telomere decreases after each round of DNA replication in normal cells. The loss of telomere occurs as the terminal DNA sequence occupied by the RNA primer towards the end of the lagging strand (Richter et al., 2007) cannot be replicated by DNA polymerase (though other factors such as oxidative stress may also contribute). A high copy number of DNA sequence repeats may ensure that shortening affects telomeric DNA primarily and avoid deleting important genetic information. Further, it has been suggested that the progressive loss of terminal DNA may continue till it reaches a specific length that activates cell senescence.
The progressive shortening of telomeres can be reversed through telomerase, which regenerates repeat sequences. Telomerase is comprised of telomerase RNA (TERC), telomerase reverse transcriptase (TERT) and several associating proteins (Venteicher et al., 2008; Xu et al., 2016). To extend shorter telomeres, TERT uses TERC’s template to add sequence repeats at the 3' end. Whereas most normal somatic cells express little telomerase, TERT activity is up-regulated in continuously dividing stem cells and ectopically expressing hTERT immortalizes non-stem cells, indicating that telomeres may affect cells’ lifespan. Consistently, an elevated telomerase activity was observed in mostf cancer specimens (Kim et al., 1994).
The antimetabolite drug 6-thioguanine has been used to treat acute myelogenous leukemia and chronic myelogenous leukemia. Its anticancer property requires activation via conversion to thioguanosine diphosphate (TGDP) or thioguanosine triphosphate (TGTP) catalyzed by hypoxanthine-guanine phosphoribosyltransferase and kinases, and the conversion to deoxyribosyl analogues by ribonucleotide reductase. Its cytotoxicity is associated with the incorporation of 6-thioguanine nucleotides into DNA during S phase to interfere with replication or into RNA to impair protein synthesis.
The impact of disrupting telomere on the growth of hTERT-expressing human cancer cells was examined. Treatment with 6-thio-2’-deoxyguanosine led to its incorporation into telomeres, rendering them dysfunctional. It resulted in the selective death of lung cancer cells expressing telomerase (Mender et al., 2015), therapy-resistant melanoma (Zhang et al., 2018) and pediatric brain tumor (Sengupta et al., 2018). The results point to the potential utility of 6-thio-2’-deoxyguanosine as a novel telomerase-dependent anti-cancer therapeutic.
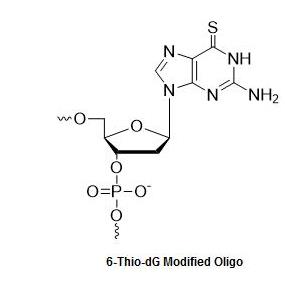
Bio-Synthesis, Inc. provides extensive options for the application of various modified nucleosides for research or therapy purposes. For instance, with 6-thio-2'-deoxyguanosine (6-thio dG), we offer oligonucleotide modification to selectively conjugate to DNA binding proteins to study their interaction. For bridged nucleic acid (BNA), we have acquired license from BNA Inc. of Osaka, Japan, to supply BNA-oligonucleotide to Bound Therapeutics LLC. to develop a novel peptide-miroRNA therapeutic for triple negative breast cancer.
https://www.biosyn.com/oligonucleotideproduct/6-thio-2%27-deoxyguanosine,6-thio-dg-oligonucleotide-modification.aspx
References
Alberts B. Molecular Biology of the Cell. Sixth edition. (2015). Garland Science, Taylor and Francis Group, New York.
Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. (1994). Science 266:2011-5. PMID: 7605428 DOI: 10.1126/science.7605428
Mender I, Gryaznov S, Dikmen ZG, Wright WE, Shay JW. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2'-deoxyguanosine. (2015). Cancer Discov 5:82-95. PMID: 25516420 PMCID: PMC4293221 DOI: 10.1158/2159-8290.CD-14-0609
Richter T, von Zglinicki T. A continuous correlation between oxidative stress and telomere shortening in fibroblasts. (2007) Exp Gerontol. 42:1039-42. PMID: 17869047 DOI: 10.1016/j.exger.2007.08.005
Sengupta S, Sobo SM, Lee K, Kumar SS, White AR, Mender I, Fuller C, Chow LML, Fouladi M, Shay JW, Drissi R. Induced telomere damage to treat telomerase expressing therapy-resistant pediatric brain tumors. (2018) Mol Cancer Ther 17:1504-1514. PMID: 29654065 DOI: 10.1158/1535-7163.MCT-17-0792
Venteicher A.S., Meng Z., Mason P.J., Veenstra T.D., Artandi S.E. Identification of APTases pontin and reptin as telomerase components essential for holoenzyme assembly. (2008). Cell 132:945–957. PMID: 18358808 PMCID: PMC2291539 DOI: 10.1016/j.cell.2008.01.019
Xu Y, Goldkorn A. Telomere and telomerase therapeutics in cancer. (2016). Genes (Basel) 7: 22. PMCID: PMC4929421 PMID: 27240403
Zhang G, Wu LW, Mender I, Barzily-Rokni M, Hammond MR, Ope O, et al. Induction of telomere Ddsfunction prolongs disease control of therapy-resistant melanoma. (2018). Clin Cancer Res 24:4771-4784. PMID: 29563139 PMCID: PMC6150856 DOI: 10.1158/1078-0432.CCR-17-2773