Molecular advances in the arena of biotechnology are increasingly making an impact on future biomedicine. As opposed to directly utilizing natural product-derived medicine, understanding its underlying pharmacological mechanism allows one to design more efficacious therapeutics based on the mechanism. The design could utilize readily accessible molecular tools such as synthetic peptides or oligonucleotides. By doing so, the costly endeavor required to extract medicinal agents from naturally occurring herbs, plants, or other biological materials could be bypassed. Alternatively, such tools could be exploited to deliver naturally occurring therapeutics to their intended targets to reduce side effects.
In the last several decades, the potential utility of synthetic peptides has been greatly expanded. Exploiting the intrinsic properties of peptides to adopt myriad conformations, pharmaceutical industries as well as academic institutions have been increasingly focusing on applying synthetic peptides to address previously unsolved medical needs. The advantages of utilizing peptides are multiple, including greater tissue penetration (ex. for solid tumor therapy) (Hong et al., 2000), the ability to modulate stability or overall charge through chemical modification (ex. peptide mimetics for oral delivery) (Cooper et al., 2021), the relative ease for interrogating the structure/conformation of shorter peptides than complex proteins composed of longer polypeptides via molecular modeling, etc. The recently developed FDA-approved peptidomimetic drug for the COVID-19 coronavirus (Paxlovid) represents one such example.
More importantly, the ability to synthesize peptides through organic chemistry at an industrial scale has facilitated greater than 6400 clinical trials. The scope of its potential being explored through clinical studies includes antiviral therapy, cancer radiotherapy, cancer immunotherapy, and treating complex disorders involving multiple genetic mutations. As some of these illnesses are becoming quite common, the demand for peptide therapeutics is likely to grow with an increasing number of elderly patients approaching or exceeding 90-100 years of age globally (ex. >3.4 million centenarians expected by 2050) (Cho et al., 2020).
On another front, the potential of utilizing synthetic peptides to address the problem of targeted drug delivery has been actively explored. Critical to this undertaking is the ability to chemically modify amino acids comprising the peptide during the synthesis at specific residues. These include phosphorylation, acetylation, amidation, citrullination, succinylation, PEGylation, etc. Equally important is the ability to conjugate peptides to a variety of agents to improve their pharmacological properties. Multiple candidate synthetic peptides have been discovered in the past utilizing phage-display technology to target normal tissues or cancerous tissues (ex. RGD, NGR, HN-1 PEP-1, etc.)
With the refinement of the delivery system, further improvements to meet specific pharmacokinetic and/or pharmacodynamic requirements for treating complex disorders are being sought. For treating diseases such as diabetes, persistent dosing of drugs may be necessary to achieve the desired therapeutic outcome. Here, the traditional schedule consisting of a singular injection (resulting in an early burst of drug dose) may not be optimal due to the eventual depletion of drugs in circulation. To meet such requirements, pharmaceutical researchers have been bioengineering ways through which a sustained release of drugs could be attained following the injection. In principle, the ‘long-acting drugs could be achieved by delaying the access of injected drugs to blood vessels from the site of injection (ex. subcutaneous tissue)—through the formation of insoluble aggregates, for instance.
An alternate method to attain persistent dosing is to compartmentalize drugs in a vehicle that allows a controlled release of the enclosed drugs while in circulation. The rapid elimination could potentially be avoided by designing vehicles whose hydrodynamic diameter exceeds the threshold for renal clearance. One such example is hydrogel comprised of synthetic peptides. Hydrogels are artificial 3-dimensional structures formed by synthetic or natural biopolymers that retain a large amount of water. It has been used for a variety of biomedical applications, including tissue implants, tissue engineering/regeneration, biosensor, etc. (Chyzy et al., 2022). To date, ~30 hydrogels have been approved by U.S. FDA for clinical use (Mandal et al., 2020).
Hydrogels self-assembled from synthetic peptides have also been utilized for drug delivery (Nguyen et al, 2011). The basic principle encompasses the formation of fibers from peptides, followed by their cross-linking to achieve gelation, the extent of which can be regulated (ex. by light). The gelation process is sensitive to multiple parameters, ex. pH, chirality, pie-pie stacking (interaction of aromatic groups), peptide sequence, etc. Hydrogels with a defined mesh size will allow a slower release of larger drugs than smaller-sized drugs. However, the process of manufacturing hydrogels with a defined mesh size via the self-assembly of peptides has been difficult to control (Namblar et al., 2022).
An innovative method is to utilize an affinity-based method to control the release of drugs. For peptide-based systems, commonly used interactions include electrostatic, hydrophobic, reversible covalent bonds (ex. disulfide bonds), etc. Upon identifying the proper binding entity for the drug, it can be engineered into the amino acids used for peptide synthesis. To release drugs, the electrostatic interaction of drugs with charged residues (ex. glutamic acid, aspartic acid, arginine) could be disrupted by altering pH or ionic strength.
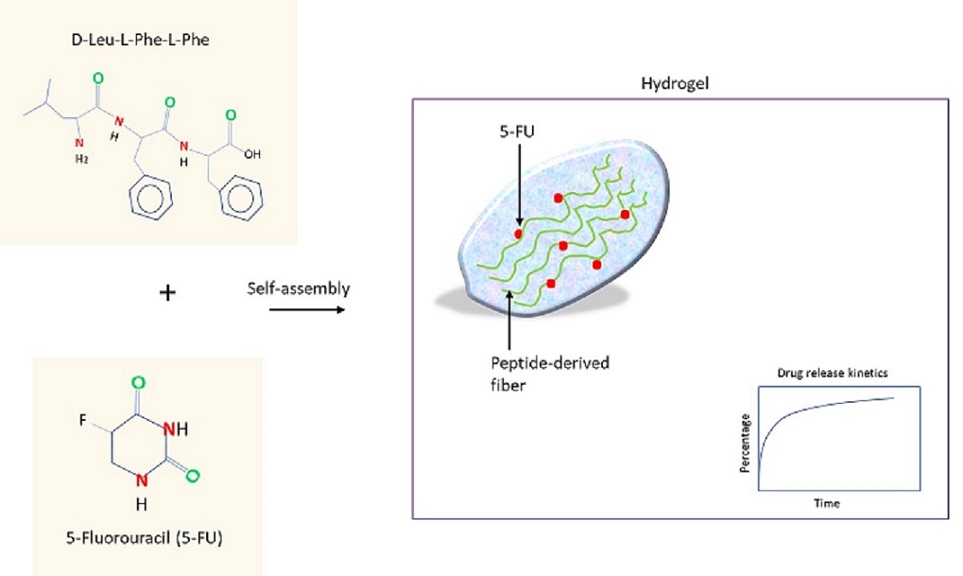
Skin cancer is the most commonly diagnosed cancer in the U.S.; among the subtypes, invasive melanoma accounts for the majority of incurred deaths (~7650 death expected in 2022). (Cancer Facts & Figures 2022 ) 5-fluorouracil (5-FU) is an antimetabolite drug used to treat skin cancer and it functions by inhibiting thymidylate synthetase, which converts dUMP to dTMP to support DNA replication (Longley et al., 2003). To resolve multiple outstanding issues such as shorter half-life or dosage variability, investigators at the University of Trieste (Italy) have developed a supramolecular hydrogel comprised of a tripeptide and the drug (Parisi et al., 2019). Intriguingly, the self-assembly by the tripeptide D-Leu-Phe-Phe occurred in the presence of 5-FU. Molecular dynamics simulation study revealed that the pie-pie stacking between the ring structure of 5-FU and the phenylalanine may underlie the drug-to-peptide interaction, which lasted transiently (~100 nanoseconds). The majority of the contained drug was released within the first hour.
The key to preventing an epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogs (over ~200) including bridged nucleic acid (BNA) in addition to mRNA synthesis. A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as to conjugate to peptides or antibodies. It provides custom conjugation of small molecules such as chemical drugs, metabolites and labeled compounds with synthetic or natural polymers (enzymes, peptide, protein, oligonucleotide, antibody, dendrimer, nanoparticle, etc). It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, the third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. It has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple-negative breast cancer. The BNA technology provides superior, unequaled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides. For therapeutic consideration, peptide synthesis or modifications may include labeling, conjugation, cyclization, incorporation of unusual amino acids, and modification of side chain and backbone.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/mrna.aspx
https://www.biosyn.com/bioconjugation.aspx
https://www.biosyn.com/tew/Design-Guidelines-for-BNA-based-Oligonucleotide-Probes.aspx#!
Peptide Modifications, Modified Peptide Synthesis - Bio-Synthesis (biosyn.com)
https://www.biosyn.com/tew/development-of-a-therapeutic-peptidomimetic-inhibiting-main-protease-to-reduce-the-level-of-rdrp-polymerase-that-replicates-covid-19-coronavirus-genome.aspx
https://www.biosyn.com/tew/peptide-therapeutics-target-dynamic-protein-to-protein-interaction-underlying-human-diseases-such-as-hypertension-cancer-alzheimers-disease-and-potentially-covid-19.aspx
References
Cho J, Hirose N, et al. Caregiving centenarians: Cross-national comparison in Caregiver-Burden between the United States and Japan. Aging Ment Health. 24: 774-783 (2020). PMID: 30596257
Chyzy A, Plonska-Brzezinska ME, et al. Microwave-Assisted Synthesis of Modified Glycidyl Methacrylate-Ethyl Methacrylate Oligomers, Their Physico-Chemical and Biological Characteristics. Molecules. 27:337 (2022). PMID: 35056652
Cooper BM, Spring DR, et al. Peptides as a platform for targeted therapeutics for cancer: peptide-drug conjugates (PDCs). Chem Soc Rev. 50:1480-1494 (2021). PMID: 33346298
Hong FD, Clayman GL. Isolation of a peptide for targeted drug delivery into human head and neck solid tumors. Cancer Res. 60:6551-6 (2000). PMID: 11118031
Longley DB, Johnston PG, et al. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 3:330-8 (2003). PMID: 12724731
Mandal A, Mitragotri S., et al. Hydrogels in the clinic. Bioeng Transl Med. 5:e10158 (2020). PMID: 32440563
Nambiar M, Schneider JP. Peptide hydrogels for affinity-controlled release of therapeutic cargo: Current and potential strategies. J Pept Sci. 28: e3377 (2022). PMID: 34747114
Nguyen LH, Linse KD, et al. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials. 32:1327-38 (2011). PMID: 21067807
Parisi E, Marchesan S., et al. Supramolecular Tripeptide Hydrogel Assembly with 5-Fluorouracil. Gels. 5 :5 (2019). PMID: 30691142