The unprecedented emergence of a pandemic caused by COVID-19 coronavirus has taken a toll socially as well as economically. Its impact has been felt globally as the virus moved on from continent to continent irrespective of the economic or technological divide. The speed with which it spread caught many in the scientific community off guard given the extent of information and experience acquired from studying closely related coronaviruses previously. It is also testing the capability of current molecular biological methods to halt its advance.
One critical arm of the preventive strategy lies in developing a diagnostic means to accurately identify the individuals infected by COVID-19. The underlying rationale is to segregate the infected to limit further exposures to reduce its propagation. Given the steady decline in mortality rate resultant from the increase in diagnosed cases and the potential for the emergence of other related viruses in the future, the long-term therapeutic efficacy of the approach is continually being assessed.
The ‘gold-standard’ of the COVID-19 diagnostic methods has been RT-PCR (reverse transcriptase-polymerase chain reaction) to detect the presence of the viral RNA for sensitivity and reliability. Additionally, alternate amplification methods based on RT-LAMP (reverse transcriptase-loop mediated isothermal amplification) are being utilized for practicality and point-of-care. As both methods have been extensively cultivated to meet expediency, the rate limiting step for the genetic diagnosis has shifted to the preceding RNA extraction step. This is not unexpected given the widely varying types of specimen from which COVID-19 RNA needs to be isolated, which include nasal swab, pharyngeal swab, sputum, saliva, oral fluid, bronchoalveolar lavage (BAL), etc.
Furthermore, the source(s) of viral RNA of COVID-19 could be diverse—i.e. mature viruses produced by infected cells, cells harboring the assembled viruses, cells with virus RNA undergoing replication, viral RNA released from ruptured/dying infected cells, etc. The integrity of the viral RNA extracted is not known as the current diagnostic methods fall short of testing its functionality. To isolate viral RNA from within cells, one needs to disrupt the cell membrane as well as the viral envelope. Although guanidine-based extraction method is effective, its adverse effects (irritating) may not be suitable for a wider usage. Other factors to consider are costs as well as availability as the supply of RNA extraction kits could be dwindled by the global needs—ex. as it may have been the case for the silica-fitted columns used in purifying nucleic acids.
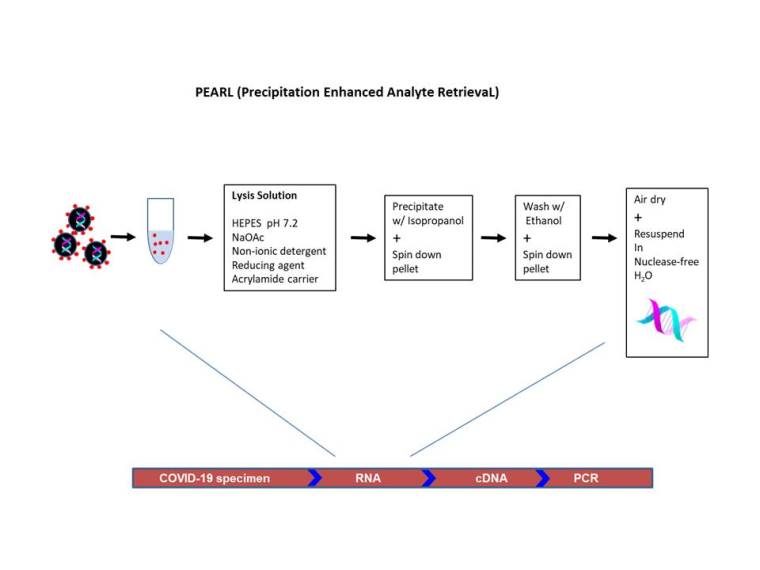
To avoid having to rely on suppliers for RNA extraction kits as well as to simplify the extraction steps, the investigators at the University of California at Santa Barbara (USA) have modified the protocol (Ponce-Rojas et al., 2020). In their protocol (called ‘PEARL’ for Precipitation Enhanced Analyte RetrievaL, 30 min prep), cells were lysed using a solution containing the non-ionic (nondenaturing) detergent IGEPAL CA-630, which structurally resembles NP-40. As the detergent is non-ionic, it may not lyse nuclear membrane (to release genomic DNA). The solution also contained linear polyacrylamide carrier to facilitate the precipitation of the material consisting of both nucleic acids (both RNA and DNA according to the report) and proteins. After washing with ethanol, the precipitated material was dissolved in nuclease-free water and used to amplify COVID-19 N1 RNA and endogenous RNase P mRNA via RT-PCR, yielding results comparable to that obtained with a commercially available kit.
Linear polyacrylamide (LPA) carrier is a commonly used non-nucleotide based co-precipitating agent that does not interfere with enzymatic reaction (ex. PCR), spectrophotometry, electrophoresis, etc., which helps to isolate minute or trace amounts of DNA or RNA via forming a visible pellet (Gaillard et al., 1990; Strauss et al., 1984). As the ability to purify DNA/RNA through precipitation is central to most molecular biological applications, LPA carrier has gained widespread usage (Li et al., 2020), which include oncology studies, and is commercially available through various biotech vendors.
The key to preventing epidemic is the ability to diagnose the infected early to preempt further propagation. For this, Bio-Synthesis, Inc. provides primers and probes (as well as synthetic RNA control) for COVID-19 diagnosis via RT-PCR assay. For medicinal chemistry, it specializes in peptide synthesis, characterization, modification, purification to generate various peptide-based building blocks as well as pharmaceutical intermediates—in addition to peptide libraries, peptide arrays, peptidomimetics. Antibody purification, characterization/quantification, modification and labeling are also offered. It specializes in oligonucleotide modification and provides an extensive array of chemically modified nucleoside analogues (over ~200) including bridged nucleic acid (BNA). A number of options are available to label oligonucleotides (DNA or RNA) with fluorophores either terminally or internally as well as conjugate to peptides. It recently acquired a license from BNA Inc. of Osaka, Japan, for the manufacturing and distribution of BNANC, a third generation of BNA oligonucleotides. To meet the demands of therapeutic application, its oligonucleotide products are approaching GMP grade. Bio-Synthesis, Inc. has recently entered into collaborative agreement with Bind Therapeutics, Inc. to synthesize miR-21 blocker using BNA for triple negative breast cancer. The BNA technology provides superior, unequalled advantages in base stacking, binding affinity, aqueous solubility and nuclease resistance. It also improves the formation of duplexes and triplexes by reducing the repulsion between the negatively charged phosphates of the oligonucleotide backbone. Its single-mismatch discriminating power is especially useful for diagnosis (ex. FISH using DNA probe). For clinical application, BNA oligonucleotide exhibits lesser toxicity than other modified nucleotides.
https://www.biosyn.com/oligo-flourescent-labeling.aspx
https://www.biosyn.com/tew/Speed-up-Identification-of-COVID19.aspx
https://www.biosyn.com/covid-19.aspx
https://www.biosyn.com/peptide-synthesis.aspx
https://www.biosyn.com/tew/Potential-Peptide-Targets-for-a-COVID-19-Vaccine.aspx
References
Gaillard C, Strauss F. Ethanol precipitation of DNA with linear polyacrylamide as carrier. Nucleic Acids Res.18:378 (1990). PMID: 2326177
Li Y, Chen S, Liu N, Ma L, Wang T, Veedu RN, et al. A systematic investigation of key factors of nucleic acid precipitation toward optimized DNA/RNA isolation. Biotechniques. 68:191-199 (2020).
Ponce-Rojas JC, Costello JS, Proctor DA, Kosik KS, Wilson MZ, Arias C, Acosta-Alvear D. A fast and accessible method for the isolation of RNA, DNA, and protein to facilitate the detection of SARS-CoV-2. bioRxiv (2020)
Strauss F, Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell 37, 889-901 (1984). PMID: 6540146